A new species of Phrynoglossus Peters, 1867; Dicroglossidae) from southeastern Bangladesh, with comments on the genera Occidozyga and Phrynoglossus
- Published
- Accepted
- Received
- Academic Editor
- Nikolay Poyarkov
- Subject Areas
- Biodiversity, Conservation Biology, Ecology, Taxonomy, Zoology
- Keywords
- Amphibia, Systematics, Phylogeny, Puddle frog, Conservation, Species complex, Bioacoustic, Morphology, Anura
- Copyright
- © 2021 Trageser et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. A new species of Phrynoglossus Peters, 1867; Dicroglossidae) from southeastern Bangladesh, with comments on the genera Occidozyga and Phrynoglossus. PeerJ 9:e11998 https://doi.org/10.7717/peerj.11998
Abstract
We describe a new cryptic species of Phrynoglossus from Chattogram Division, Bangladesh based on an integrative taxonomic analysis based on morphology, phylogenetics, and bioacoustics which unambiguously support the placement of the species in the genus Phrynoglossus. We also present a compilation of published morphological characters for all twelve Phrynoglossus species and two species of Occidozyga as well as comments on taxonomy, morphology, and geographic distribution for the two genera. The new species is found to be most morphologically similar to P. martensii, however a provided set of character states visibly differentiates these two species. Finally, habitat for Phrynoglossus swanbornorum sp. nov. is highly fragmented and faces imminent threats from development and agriculture, and although it is confirmed to occur within government protected areas in the southeastern region of Bangladesh, few wildlife regulations are enforced within them. Thus, following IUCN criteria, we consider the new species as Endangered based on criteria B1ab(i,ii,iii,iv) + 2ab(i,ii,iii,iv).
Introduction
Phrynoglossus (Peters, 1867) is a widely distributed genus in South and Southeast Asia whose occurrence is reported to reach as far west as West Bengal (India; see discussion), east to southern Jiangxi and eastern Fujian (China), and southeast to Java, Bali, Flores, Sulawesi, and the Philippines (Frost, 2021; Günther, 1864). They are semi-aquatic frogs with relatively small and stocky bodies, and are often called Javan, puddle, seep, or floating frogs due to their preference for lentic habitats; though they are not entirely restricted to them. Previous diagnoses of Phrynoglossus have characterized the genus as having the following combination of traits: small size, stocky habitus, short hind limbs, an indistinct or moderately distinct tympanum, no vomerine teeth, a fleshy tongue that is pointed or rounded, a flattened snout, horizontal pupils, dorsum with scattered tubercles, skin covered by an extensive mucosome, throat lining uniformly grey, tips of fingers and toes slightly swollen, length of finger I equal to II, enlarged and rounded toe tips lacking grooves, a semiaquatic life style, and inguinal amplexus (see discussion; Taylor, 1962; Iskandar, 1998; Sailo et al., 2009; Inger et al., 2017; Poyarkov et al., 2020; Köhler et al., 2021. In contrast, Occidozyga (Occidozyga Kuhl & Van Hasselt, 1822) has recently been characterized as being distinct from Phrynoglossus by exhibiting a slender worm-like tongue, pointed tips of fingers and toes, concealed tympanum, dryer skin not covered by extensive mucous, whitish throat lining with a longitudinal brown stripe, a fully aquatic lifestyle, and axillary amplexus (see discussion; Köhler et al., 2021).
The generic assignments of Phrynoglossus and Occidozyga have had a polyonymous history (Frost, 2021), and are currently recognized by some as monophyletic sister-taxa (Köhler et al., 2021). However, phylogenetic relationships within the clade are far from being resolved (Dubois, Ohler & Biju, 2001; Frost et al., 2006; Sailo et al., 2009; Iskandar, Arifin & Rachmansah, 2011; Inger et al., 2017; Bogisich, 2019; Chan et al., 2021; Köhler et al., 2021). Phrynoglossus currently comprises 12 species (Frost, 2021), with P. martensii (Peters, 1867) representing the type species and Occidozyga comprises two species with Occidozyga lima (Gravenhorst, 1829) representing the type species. A recent study resulted in the synonymy of P. laevis vittata (Andersson, 1942) with P. martensii (Poyarkov et al., 2020), and two unpublished theses have highlighted the presence of undescribed cryptic lineages within the O. lima complex (Chan, 2013) and the P. martensii complex (Bogisich, 2019). Nonetheless, only one new species of either Phrynoglossus and Occidozyga has been described in the past 60 years (i.e., Iskandar, Arifin & Rachmansah, 2011), until 2021 when two additional species were described (Köhler et al., 2021; Matsui et al., 2021).
Herein, we describe a new species of Phrynoglossus from the lowland, semi-evergreen forests of southeast Bangladesh—a region with dense human populations. Patches of mature forest habitat remain throughout its known range, though these are highly fragmented and degraded as a result of unsustainable agricultural practices and ongoing deforestation (Gain, 2002; Kabir & Muzaffar, 2002). The new species is both morphologically and genetically distinct from its congeners, despite substantial morphological overlap with P. martensii. Lastly, we provide a compilation of published morphological characters for all 14 Phrynoglossus and Occidozyga species with comments on taxonomy, morphology, and geographic distribution for the genera.
Materials & Methods
Ethics statement
Specimen collection protocols and animal use were approved by the Department of Zoology of Jagannath University. Field work was conducted under permit 22.01.0000.101.23.2019.1922., issued by the Bangladesh Forest Department. The study was carried out in accordance with the guidelines for use of live amphibians and reptiles in field and lab research (Beaupre et al., 2004), compiled by the American Society of Ichthyologists and Herpetologists (ASIH) Herpetologists’ League HL, and the Society for the Study of Amphibians and Reptiles (SSAR).
Taxonomy and species concept
Puddle frog generic placement follows the taxonomy proposed by Köhler et al. (2021). For recognizing species, we adhere to the General Species Concept (De Queiroz, 2005; De Queiroz, 2007). Under this concept, the only necessary property for an entity to be a recognized as a species is that it corresponds to a temporal segment of a metapopulation lineage evolving separately from other lineages. Independent evolution generates diagnostic traits detectable in a species’ morphology, vocalizations, behavior, and genetics.
Study areas
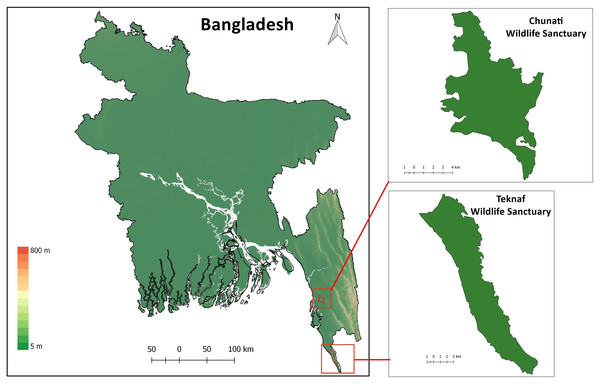
Chunati Wildlife Sanctuary (CWS) is situated in Chattogram District of Chattogram Division (formerly known as Chittagong Division) in southeast Bangladesh (21.900000 N 92.133333 E; Fig. 1). It was established as a Wildlife Sanctuary in 1986 with an area of 7,764 ha and consists of semi-evergreen forest 30 to 90 m above sea level, with narrow valleys and streamlets caused by broken hillocks orientated in a north-south direction. The area experiences a moist, subtropical climate with a low range of temperature and humidity variation with November to February being the coldest and driest months. The sanctuary has experienced substantial degradation in recent years due to illicit felling of trees, cultivation of betel-leaf, and creation of rice paddies as part of its management scheme. As a result, the sanctuary has lost most of its natural forest (Gain, 2002; Kabir & Muzaffar, 2002). Forest coverage in 2015 was reduced to 60% (Rahman et al., 2016). Trees such as Dipterocarpus sp., Artocarpus sp., Albizia sp., Quercus sp., Syzygium sp., and Lagerstroemia sp. still occur in CWS although they are mostly found as isolated individuals or in small, fragmented patches.
Teknaf Wildlife Sanctuary (TWS) is situated in Cox’s Bazar District of Chattogram Division, near Myanmar (20.887020 N, 92.298954 E; Fig. 1; Rosario, 1997). It was initially granted formal protection in 1983 as a Game Reserve and its total area is approximately 11,651 ha (Green, 1987). While TWS once supported primary evergreen and semi-evergreen forests, these forests have now been largely replaced with human-modified landscapes and only degraded forest remains (Alam et al., 2015). The reserve is longitudinally narrow, running roughly 28 km in a north-south direction and 3–5 km east–west (Uddin et al., 2013) with linear hill tracts reaching as high as 700 m above sea level. Between 130 and 940 mm of rain usually falls in May through October which causes numerous tributaries of the Naf river to flow through TWS mainly during the monsoon season. Average temperature in TWS can range from 15 °C to 32 °C (BBS, 2011).
Figure 1: Map of Bangladesh with insets of study areas Chunati Wildlife Sanctuary and Teknaf Wildlife Sanctuary.
Specimen collection
Four adult males and one adult female were collected along a roadside, adjacent to mature forest, near Chunati Wildlife Sanctuary (21.93029 N, 92.06316 E) on June 30, 2019. Collected specimens were euthanized using 20% benzocaine and were fixed in 95% for five hours and preserved in 70% EtOH. Muscle samples were preserved in 95% EtOH. Color of live specimens was photographed and recorded in the field during specimen collection. All specimens collected were deposited in the Shahid Rafique Special Specimen Collection (SRSSC), Department of Zoology, Jagannath University, Dhaka.
Morphological data
Morphometric measurements were taken of the specimens with digital calipers (to the nearest 0.01 mm) after preservation. The following measurements were taken (Iskandar, Arifin & Rachmansah, 2011): snout-vent length (SVL): from tip of snout to vent; head length (HL): distance between tip of snout to the rear of the mandible; head width (HW): at angle of jaw; eye diameter (ED): horizontal diameter of the eye; tympanum diameter (TD): maximum diameter of the tympanum; eye-nostril distance (EN): distance between anterior canthus of eye and the nostril; snout length (SL): from anterior canthus of eye to tip of snout; nostril-snout distance (NS): distance from the nostril to the tip of the snout; interorbital distance (IOD): least distance between proximal edges of upper eyelids; internarial distance (IND): least distance between nostrils; upper eyelid width (UEW): distance of the upper eyelid measured from inner edge to outer edge; thigh length (TL): distance from the middle of vent to knee; shank length (SHL): distance between knee and heel; foot length (FOL): from the base of the inner metatarsal tubercle to the tip of toe IV; hand length (HAL): from the base of the outer palmar tubercle to the tip of finger IV; lengths of 1st to 4th fingers (FL I to FL IV): from the base of the palm to the tip of the respective finger; lengths of 1st to 5th toes (TL I to TL V): from the base of proximal subarticular tubercle to tip of the respective toe.
DNA extraction and amplification
DNA was extracted from muscle tissue using a standard protocol following Vences et al. (2012). A small section of thigh muscle tissue was excised from five specimens for extraction, from which, mitochondrial 16S ribosomal RNA gene was amplified. The PCR amplification and sequencing of the 16S rRNA gene were done following Palumbi et al. (1991) and Bossuyt et al. (2004) respectively. Primers 5′-GCCTGTTTATCAAAAACAT-3′(16Sar-L) and 5′-CCGGTCTGAACTCAGATCACGT-3′(16Sbr-H) were used as forward and reverse primers for 16S (Palumbi et al., 1991). PCR amplifications were performed in a 20 µl reaction volume containing 10 µl Master Mix, 1 µl T DNA (concentration 25–65 ng/µl), 1 µl forward primer (concentration 10–20 pMol), 1 µl reverse primer (concentration 10–20 pMol), and 7 µl nuclease-free water. Performed cycling conditions were as follows: an initial denaturing step at 95 °C for 3 min; 40 cycles of denaturing at 95 °C for 30 s, annealing at 50 °C for 30 s, extending at 72 °C for 45 s, and a final extending step of 72 °C for 5 min. The amplified product was sequenced at 1st Base Laboratories, Malaysia.
Phylogenetic analyses
Homologous sequences were obtained from GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (Table 1) for all species of Phrynoglossus and Occidozyga with the exception of four species with no available 16S sequences in Genbank—P. floresianus, P. celebensis, P. semipalmatus and P. tompotika. Limnonectes limborgi (Sclater, 1892) was selected as the outgroup to align with the analysis of Köhler et al. (2021). Sequences were aligned using the MUSCLE tool in MEGA 7 (Kumar, Stecher & Tamura, 2016) and alignments were checked visually. Alignment gaps were treated as missing data. The best substitution model, General Time Reversible with proportion of invariable sites and gamma distribution (GTR+I+G), was selected using the Akaike Information Criterion (AIC) and (GTR+G) Bayesian information criteria (BIC) in jModelTest v2.1.2. Maximum likelihood phylogenetic analyses were performed using the RAxML v4.0 Geneious plugin (Stamatakis, 2006) with 1,000 bootstrap replicates. Bayesian phylogenetic inference analysis was performed in MrBayes 3.2.4 (Ronquist et al., 2012). We performed a MCMC Bayesian analysis that consisted of two simultaneous runs of 1 million generations and sampled every 100 generations. The first 25% of the sampled trees were discarded as burn-in and the remaining trees were used to create a consensus tree and to estimate Bayesian posterior probabilities (BPPs). The trees were visualized and edited in FigTree 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree). Additionally, 16S pairwise genetic distances (uncorrected p) for six Phrynoglossus species including Phrynoglossus sp. nov. were calculated using MEGA 7.0 (Kumar, Stecher & Tamura, 2016).
| Species | Location | Voucher |
GenBank 16S rRNA accession numbers |
Source | |
|---|---|---|---|---|---|
| 1 | P. swanbornorumsp. nov. | Chattogram, Bangladesh | JnUZool-A0719 | MN705433 | This study |
| 2 | P. swanbornorumsp. nov. | Chattogram, Bangladesh | JnUZool-A0819 | MN705434 | This study |
| 3 | P. swanbornorumsp. nov. | Chattogram, Bangladesh | JnUZool-A0919 | MN705435 | This study |
| 4 | P. swanbornorumsp. nov. | Chattogram, Bangladesh | JnUZool-A1019 | MN705436 | This study |
| 5 | P. swanbornorumsp. nov. | Chattogram, Bangladesh | JnUZool-A1117 | MN705437 | This study |
| 6 | P. myanhessei | Thanlyin, Yangon, Myanmar | SMF 103797 | MW217501 | Köhler et al. (2021) |
| 7 | P. myanhessei | Thanlyin, Yangon, Myanmar | SMF 103798 | MW217502 | Köhler et al. (2021) |
| 8 | P. myanhessei | Thanlyin, Yangon, Myanmar | SMF 103800 | MW217503 | Köhler et al. (2021) |
| 9 | P. myanhessei | Yangon, Myanmar | USNM:Herp:587107 | MG935920 | Mulcahy et al. (2018) |
| 10 | P. myanhessei | Bago, Myanmar | USNM:Herp:587105 | MG935916 | Mulcahy et al. (2018) |
| 11 | P. myanhessei | Yangon, Myanmar | USNM:Herp:587395 | MG935918 | Mulcahy et al. (2018) |
| 12 | P. myanhessei | Yangon, Myanmar | USNM:Herp:587402 | MG935917 | Mulcahy et al. (2018) |
| 13 | P. martensi | Myanmar | USNM:Herp:586940 | MG935942 | Mulcahy et al. (2018) |
| 14 | P. martensi | Myanmar | USNM:Herp:586942 | MG935941 | Mulcahy et al. (2018) |
| 15 | P. martensi | Myanmar | USNM:Herp:586943 | MG935940 | Mulcahy et al. (2018) |
| 16 | P. martensi | Myanmar | USNM:Herp:586930 | MG935939 | Mulcahy et al. (2018) |
| 17 | P. martensi | Myanmar | USNM:Herp:586931 | MG935938 | Mulcahy et al. (2018) |
| 18 | P. martensi | Myanmar | USNM:Herp:586937 | MG935932 | Mulcahy et al. (2018) |
| 19 | P. martensi | Myanmar | USNM:Herp:586938 | MG935931 | Mulcahy et al. (2018) |
| 20 | P. martensi | Myanmar | USNM:Herp:586939 | MG935930 | Mulcahy et al. (2018) |
| 21 | P. martensi | Myanmar | USNM:Herp:586941 | MG935929 | Mulcahy et al. (2018) |
| 22 | P. sp. | Yangon, Myanmar | MBM-JBS19932 | MG935921 | Mulcahy et al. (2018) |
| 23 | P. sp. | Yangon, Myanmar | USNM:Herp:587389 | MG935919 | Mulcahy et al. (2018) |
| 24 | P. sp. | Yangon, Myanmar | USNM:Herp:587386 | MG935914 | Mulcahy et al. (2018) |
| 25 | O. lima | Myanmar | USNM:Herp:586925 | MG935926 | Mulcahy et al. (2018) |
| 26 | O. lima | Myanmar | USNM:Herp:586927 | MG935928 | Mulcahy et al. (2018) |
| 27 | O. lima | Myanmar | USNM:Herp:586926 | MG935927 | Mulcahy et al. (2018) |
| 28 | O. lima | Myanmar | USNM:Herp:586924 | MG935925 | Mulcahy et al. (2018) |
| 29 | O. lima | Sagaing, Myanmar | USNM:Herp:520376 | MG935924 | Mulcahy et al. (2018) |
| 30 | O. lima | Mandalay, Myanmar |
MBM-JBS5405 | MG935923 | Mulcahy et al. (2018) |
| 31 | O. berbezus | Malaysia:Matang | KUHE:17327 | LC593607 | Matsui et al. (2021) |
| 32 | P. magnapustulosus | Thiland | GK_7395 | MW217488 | Köhler et al. (2021) |
| 33 | P. magnapustulosus | Thiland | GK_7396 | MW217487 | Köhler et al. (2021) |
| 34 | P. magnapustulosus | Thiland | GK_7916 | MW217489 | Köhler et al. (2021) |
| 35 | P. laevis | Philippines: Pasonanca, | KU 314470 | MT820168 | Chan et al. (2021) |
| 36 | P. laevis | Philippines: Pasonanca, | KU 319796 | MT820169 | Chan et al. (2021) |
| 37 | P. sumatranus | Selangor, Malaysia | FRIM 1132 | MT820181 | Chan et al. (2021) |
| 38 | P. sumatranus | Selangor, Malaysia | FRIM 1133 | MT820182 | Chan et al. (2021) |
| 39 | P. sumatranus | Selangor, Malaysia | FRIM 1936 | MT820183 | Chan et al. (2021) |
| 40 | P. diminutivus | Philippines: Pasonanca, | KU 321225 | MT820199 | Chan et al. (2021) |
| 41 | P. diminutivus | Philippines: Pasonanca, | KU 321226 | MT820200 | Chan et al. (2021) |
| 42 | P. diminutivus | Philippines: Pasonanca, | KU 321227 | MT820201 | Chan et al. (2021) |
| 43 | P. baluensis | Sabah, Myanmar | FMNH 242747 | DQ283143 | Frost et al. (2006) |
| 44 | Limnonectes limborgi | Myanmar | GK_7110 | MW217495 | Köhler et al. (2021) |
Call recording and analysis
Call analysis is based on a single recording of advertisement calls from a single male Phrynoglossus sp. nov. obtained by SJT on June 30, 2019 at 20:00 h during light rain and ambient air temperature measuring 30.1 °C. Advertisement call recording was made with an LG V30 smartphone with sampling rate of 44.1 kHz and 32-bit resolution. The smartphone was placed approximately 1.5 m from the calling male.
The original call recording file was converted from .m4a format to .wav format using Adobe® Audition® (version 13.0.1.35; Adobe Inc., San Jose, CA, USA). Adobe Audition sound removal process was used to generate a recording to facilitate measuring temporal variables. Sound removal was applied with the following settings: sound model complexity 60, sound refinement passes 150, content complexity 60, and content refinement passes 150. Sound model was trained and applied with segments from 1.5–3 s and 10–11.5 s.
Call duration was defined as the length of a note. Call period was the time interval from the beginning of one note to the beginning of the next note. Call repetition rate was the inverse of note period. The number of pulses was the number of pulses in a note. Pulse rate was calculated as the number of pulses in a note divided by call duration. Call bandwidth was measured using the “Freq 5%” and “Freq 95%” measurement functions in Raven Pro 1.61. Dominant frequency was defined as the frequency with the most energy. Spectrogram configuration was set at Hann window of 512-sample window size and 256-sample hop size with 50% frame overlap and 86.1-Hz frequency grid spacing. Each call was analyzed for both temporal and spectral domains (Raven Pro 1.6.1; Cornell Lab of Ornithology, New York, USA), following the recommendations of Köhler et al. (2017).
Conservation
Extinction risk was evaluated based on IUCN Red List categories and Criteria (2012). Area of occupancy and extent of occurrence were calculated using the software, GeoCAT, with a default cell size of 2 km2.
Nomenclature
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved, and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for Phrynoglossus swanbornorum sp. nov. is: urn:lsid:zoobank.org:pub:DE8850C6-0A7B-4EA2-AC9E-69EE6EFBF754. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Results
Phylogenetic relationships of Phrynoglossus.
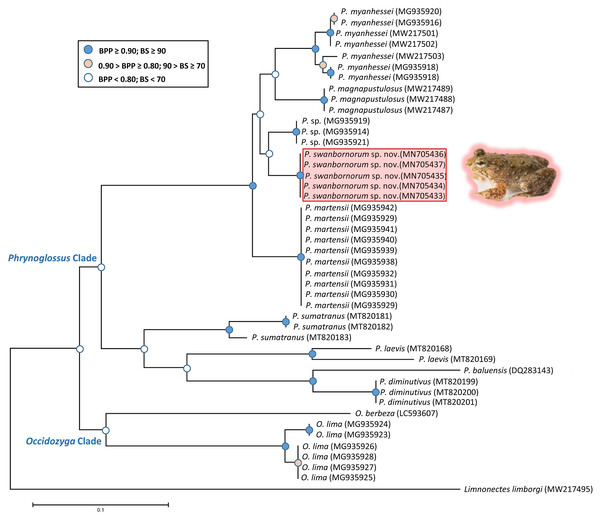
The ML and BI analyses resulted in essentially identical topologies and were integrated in the consensus tree derived from the analyses of 2001 bp of 16S rRNA gene fragments alignment (Fig. 2), in which all intraspecies nodes were sufficiently supported with the Bayesian posterior probabilities (BPP > 0.90) and the bootstrap supports (BS > 70). Most interspecies nodes had poor support (BPP < 0.80; BS < 70) with the exception of the node supporting P. myanhessei, P. magnapustulosa, P. swanbornorum sp. nov., P. sp., and P. martensii. A second major Phrynoglossus clade comprises P. sumatrana, P. laevis, P. baluensis, and P. diminutiva and is poorly supported (BPP < 0.80; BS < 70). Support for the Phrynoglossus and Occidozyga clades is similarly poorly supported (BPP < 0.80; BS < 70). Although P. martensii is morphologically similar to our new species, its genetically distinctiveness is strongly supported (BPP > 0.90; BS > 90).
The uncorrected p-distances for the 16S rRNA gene, which are interpreted as interspecific distances, were lowest between our new species and another undescribed species from Yangon, Myanmar (p = 3.9%) and P. magnapustulosa (p = 4.9%, Table 2). The highest interspecific distances were between O. lima and P. laevis (Günther, 1858) (p = 19.9%, Table 2). The average divergence (p-distance) within the new species ranged from 0.2% to 0.7% and average divergence between congeners ranged from 5.3% (P. myanhessei) to 18.3% (O. berbeza; Table 2). This level of divergence in the 16S rRNA gene is typically seen in many other frog species (Fouquet et al., 2007; Vences et al., 2005), thereby justifying the status of Phrynoglossus swanbornorum sp. nov. as a new species.
Figure 2: Consensus tree of merged Bayesian Inference and Maximum Likelihood analyses with Bayesian posterior probabilities and bootstrap supports.
Species description
Recommended vernacular name. English: Swanborn’s Puddle Frog. Bangla: “Chattgai ar gata bang,” which translates to English as “puddle frog from Chattogram.”
Holotype. (Figs. 3–5). JnUZool-A0719, adult male from a roadside ditch near Chunati Wildlife Sanctuary, Chattogram Division, Bangladesh (21.937533 N, 92.063010 E, ca. 33 m a.s.l., Fig. 1), collected on June 30, 2019 by Fahimuzzaman Nobel.
Paratypes. Three adult males (JnUZool-A0919, JnUZool-A1019, and JnUZool-A1119) and one adult female (JnUZool-A0819) with the same data and place as the holotype.
Chresonymy. Within Bangladesh, this species was first reported from southeast Bangladesh in the Teknaf peninsula (Khan, 1997; Khan, 2001), but without photographic evidence or reference to museum specimens (see Distribution).
Generic placement. The new species is assigned to the genus Phrynoglossus based on the following combination of shared adult characters: lacking vomerine teeth and exhibiting a stocky habitus, flattened snout, dorsum or flanks with scattered tubercles, short arms, nuptial pads, horizontal pupils, bony style, forked omosternum, pigmented eggs, distinct or indistinct supratympanic fold, reduced to absent metacarpal webbing, moderate to extensive metatarsal webbing, an elongated inner metatarsal tubercle, indistinct and small tympanum, feebly to moderately developed toes discs, SVL between 15 mm and 61.6 mm in length, and females are larger than males. These adult characters agree with previous descriptions of the genus (see discussion; Taylor, 1962; Iskandar, 1998; Sailo et al., 2009; Inger et al., 2017; Poyarkov et al., 2020; Köhler et al., 2021). Larval characters are unknown.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | P. swanbornorum sp. nov. | |||||||||||||||
| 2 | P. swanbornorum sp. nov. | 0.2 | – | |||||||||||||
| 3 | P. swanbornorum sp. nov. | 0.2 | 0.0 | – | ||||||||||||
| 4 | P. swanbornorum sp. nov. | 0.2 | 0.0 | 0.0 | – | |||||||||||
| 5 | P. swanbornorum sp. nov. | 0.7 | 0.5 | 0.5 | 0.5 | – | ||||||||||
| 6 | P. myanhessei | 5.3 | 5.3 | 5.3 | 5.3 | 5.3 | – | |||||||||
| 7 | P. martensii | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 | 7.3 | – | ||||||||
| 8 | Phrynoglossus sp. | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 6.4 | – | |||||||
| 9 | P. magnapustulosus | 4.9 | 4.9 | 4.7 | 4.9 | 4.9 | 6.0 | 5.9 | 6.5 | – | ||||||
| 10 | P. laevis | 15.4 | 15.4 | 15.4 | 15.4 | 15.4 | 16.8 | 14.8 | 16.2 | 16.1 | – | |||||
| 11 | P. sumatranus | 15.9 | 15.9 | 15.4 | 16.0 | 16.0 | 15.3 | 15.3 | 15.1 | 15.4 | 15.3 | – | ||||
| 12 | P. diminutivus | 18.0 | 18.0 | 17.5 | 17.9 | 17.9 | 19.6 | 19.3 | 18.5 | 18.4 | 17.8 | 16.2 | – | |||
| 13 | P. baluensis | 17.5 | 17.5 | 17.1 | 17.4 | 17.4 | 19.1 | 16.7 | 18.7 | 18.1 | 18.7 | 15.8 | 9.6 | – | ||
| 14 | O. berbeza | 17.8 | 17.8 | 18.3 | 18.2 | 18.2 | 19.2 | 192 | 18.2 | 18.9 | 17.6 | 17.6 | 16.6 | 18.8 | – | |
| 15 | O. lima | 16.2 | 16.2 | 15.8 | 16.2 | 16.2 | 17.9 | 16.9 | 15.7 | 16.4 | 19.9 | 18.0 | 18.9 | 16.8 | 19.3 | – |
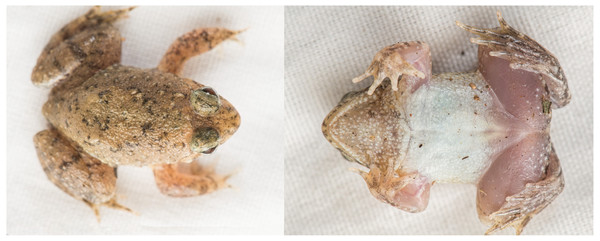
Figure 3: Photographs of live holotype JnUZool-A0719.
Figure 4: Photographs of holotype JnUZool-A0719.
(A) Dorsal aspect, in preservation (B) ventral aspect, in preservation (C) posterior aspect, in preservation (D) posterior in lateral aspect, in preservation (E) hand in dorsal aspect, in preservation (F) foot in dorsal aspect, in preservation.Figure 5: Phrynoglossus swanbornorum sp. nov. individuals.
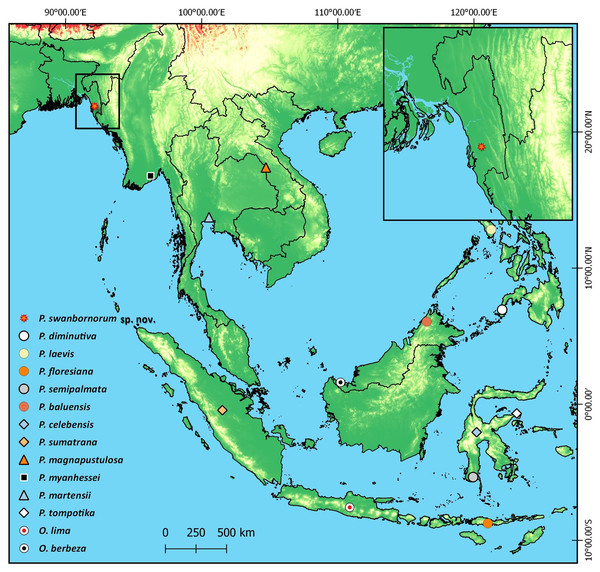
(A) Individual 1 with diamond shaped pupil, in life (B) individual 1 with ovoid shaped pupil, in life (C) individual 2 exhibiting no dorsolateral line, in life (D) individual 1 exhibiting dorsolateral line, in life.Figure 6: Type localities of all 14 species of Occidozyga and Phrynoglossus species.
Diagnosis. The new species presents the following characteristics: (1) relatively small sized Phrynoglossus (adult males = 23.15–28.56 mm SVL, adult female = 30.56 mm); (2) stocky habitus; (3) short arms relative to body size (FLL/SVL 0.19%; range 0.18−0.20%, n = 5); (4) head wider than long (HL/HW 0.73%; range 0.69−0.77%, n = 5); (5) snout slightly projecting, triangular in ventral aspect, smaller than horizontal diameter of eye, and flat in lateral aspect; (6) nostril closer to tip of snout than to eye (NS 0.48−0.51 mm, EN 0.92–109 mm); (7) brown dorsum transitioning to brownish grey on flanks with black speckling concentrated around scattered tubercles, nares, mouth, and above eyes; (8) venter uniform cream white, becoming brown with white mottling and groups of minute dark-grey flecks present in the gular region; (9) tuberculate dorsum; (10) laterally oriented eyes and nares; (11) horizontal pupils; (12) vomerine teeth absent; (13) tongue rounded without notch behind; (14) vocal sac single, median, internal, and subgular in males; (15) canthus rostralis rounded; (16) tympanum indistinct, small, and rounded; (17) supratympanic fold distinct and transverses interorbitally at the posterior edge of the eye; (18) fingertips rounded without discs; (19) nuptial pads present in males; (20) palmar tubercles present; (21) metacarpal webbing absent; (22) toe discs feebly developed; (23) inner metatarsal tubercle elongated and compressed, present at base of 1st toe; (24) fringe of skin on outer side of fifth metatarsal absent; (25) tarsal tubercle absent; (26) tarsal fold absent; (27) metatarsal webbing moderate; and (28) lateral line absent. Details of these characteristics are provided in Table 3.
Description of holotype. Adult male (JnUZool-A0719). SVL = 24.89 mm (Fig. 3; Table 3; all measurements in mm); head wider than long (HW/HL ratio 145.8%); snout triangular in ventral aspect, shorter than eye diameter (ED/SL ratio 69.5%), flat in lateral aspect; canthus rostralis rounded; loreal region slightly convex; interorbital region flat and smaller than the upper eyelid (IOD/UEW ratio 77.6%) and internarial distance (IOD/IND ratio 91.7%); nostrils rounded, directed laterally, and closer to tip of snout than to eye (NS/EN ratio 47.6%); tympanum small, rounded, close to eye, and covered by skin but with outline faintly visible (TD = 1.21); supratympanic fold distinct and transverses interorbitally at the posterior edge of the eye; eyes relatively large (ED = 3.02) and protruding; pupils horizontal, and ovoid when dilated and diamond when constricted; vocal sac single, median, internal, and subgular; numerous minute tubercles present on the anal portion and the base of the foot; fine dorsolateral ridges on shank.
Forelimb length approximately equal to hand length (FLL /HAL ratio 98.4%); relative lengths of fingers IV<II<I<III (FL I = 1.75; FL II = 1.56; FL III = 2.51; FL IV = 1.45); fingertips rounded and without disk; toe discs feebly developed; webbing between fingers absent; subarticular tubercles well developed and rounded; number of subarticular tubercles in fingers: I = 1, II = 1, III = 2, IV = 1; supernumerary tubercles indistinct; nuptial pad present.
Hind limbs long, shank longer than thigh (SHL/TL ratio 113.4%) and foot (SHL/FOL ratio 109.9%); relative toe length I<V<II<III<IV (TL I=2.10; TL II = 3.80, TL III = 5.32; TL IV = 7.23; TL V=3.63); toes with small discs and circummarginal grooves absent; webbing moderate; webbing formula (fingers: I - 2 II - III - 3 IV - V) (Figs. 4D, 4E); inner and outer metatarsal tubercles absent; subarticular tubercle present and rounded (toe: I=1, II = 1, III = 1, IV = 2, V=2); inner metatarsal tubercle elongated (1.86 mm), compressed, and present at base of 1st toe; fringe of skin on outer side of fifth metatarsal absent.
Coloration of holotype in life. In life, all specimens exhibited a brown dorsum transitioning to brownish grey on flanks with black speckling concentrated around scattered tubercles, nares, mouth, and above eyes; narrow, yellow dorsolateral stripe present or absent; forelimbs with black spots and hind limbs with black bands; dorsal tubercles on flanks tipped in brownish white to white; shanks with incomplete dark banding; venter uniform cream white; gular region brown with white mottling and scattered groups of dark-grey flecks; ventral side of feet translucent with heavy black speckling; ventral side of hands light tan with moderate black speckling; pupil ovoid when dilated and diamond when constricted; and iris has many fine, dark spots which can create indistinct reticulations which are predominantly silver and distally bordered by brown below the pupil, dark blotches in the median region, and predominantly brown with fine, dark reticulations above the pupil (Fig. 5).
Coloration of holotype ethanol. In ethanol, the pattern described above has not changed, although color has faded. Brownish tint has faded to dark grey on dorsum and light grey on tympanum; webbing coloration faded from pinkish to cream white; ventral side of hands faded to light grey; skin covering the tympanum appears thinner in preserved specimens which exaggerates the appearance of the tympanum; and other dehydration artifacts are present.
Variation and sexual dimorphism. The only preserved female (30.6 mm) is slightly larger than the largest male (28.6 mm). There are no other notable differences among the type series, however a photographed individual of Phrynoglossus swanbornorum sp. nov. from Teknaf Wildlife Sanctuary exhibits a distinct dorsolateral stripe and was documented constricting its ovoid pupil to appear diamond shaped (Figs. 5B and 5D). Variation in size and body proportions in this species are given in Table 3.
| P. martensii | P. magnapustulosus | P. myanhessei | P. swanbornorum | ||
|---|---|---|---|---|---|
| ♂7 — ♂8 | ♂8 —♂12 | ♂9 —♂6 | ♂4 —♂1 | ||
| SHL/SVL | males | 0.441–0.509 (0.479 ± 0.021) | 0.442–0.557 (0.509 ± 0.036) | 0.457–0.571 (0.506 ± 0.032) | 0.428–0.449 (0.440 ± 0.009) |
| female | 0.436–0.479 (0.462 ± 0.016) | 0.402–0.548 (0.478 ± 0.041) | 0.466–0.592 (0.500 ± 0.047) | 0.429 | |
| FL/SVL | males | 0.471–0.535 (0.491 ± 0.024) | 0.473–0.564 (0.527 ± 0.031) | 0.433–0.535 (0.476 ± 0.037) | 0.374–0.418 (0.410 ± 0.027) |
| female | 0.432–0.488 (0.468 ± 0.021) | 0.439–0.567 (0.482 ± 0.045) | 0.407–0.491 (0.449 ± 0.033) | 0.388 | |
| HL/SVL | males | 0.341–0.383 (0.359 ± 0.015) | 0.313–0.377 (0.341 ± 0.024) | 0.268–0.378 (0.330 ± 0.031) | 0.226–0.257 (0.240 ± 0.015) |
| female | 0.311–0.383 (0.344 ± 0.022) | 0.272–0.347 (0.304 ± 0.028) | 0.272–0.304 (0.289 ± 0.011) | 0.236 | |
| HW/SVL | males | 0.336–0.401 (0.357 ± 0.023) | 0.335–0.394 (0.364 ± 0.021) | 0.286–0.369 (0.338 ± 0.029) | 0.300–0.351 (0.331 ± 0.023) |
| female | 0.338–0.388 (0.351 ± 0.017) | 0.295–0.360 (0.336 ± 0.019) | 0.283–0.354 (0.312 ± 0.025) | 0.308 | |
| HL/HW | males | 0.909–1.077 (1.011 ± 0.063) | 0.855–1.030 (0.937 ± 0.058) | 0.897–1.191 (0.980 ± 0.092) | 0.685–0.758 (0.724 ± 0.029) |
| female | 0.920–1.020 (0.980 ± 0.031) | 0.779–1.057 (0.907 ± 0.098) | 0.808–1.043 (0.929 ± 0.093) | 0.765 | |
| IOD/SVL | males | 0.071–0.091 (0.086 ± 0.007) | 0.093–0.135 (0.112 ± 0.015) | 0.090–0.132 (0.107 ± 0.013) | 0.041–0.049 (0.046 ± 0.003) |
| female | 0.073–0.084 (0.077 ± 0.003) | 0.084–0.137 (0.106 ± 0.018) | 0.077–0.163 (0.104 ± 0.032) | 0.04 | |
| TYD/SVL | males | 0.042–0.068 (0.052 ± 0.010) | 0.055–0.099 (0.082 ± 0.014) | 0.029–0.066 (0.050 ± 0.012) | 0.040–0053 (0.049 ± 0.004) |
| female | 0.035–0.052 (0.043 ± 0.006) | 0.062–0.102 (0.080 ± 0.012) | 0.034–0.064 (0.051 ± 0.010) | 0.04 | |
| EYD/SVL | males | 0.109–0.138 (0.122 ± 0.010) | 0.091–0.143 (0.116 ± 0.017) | 0.094–0.140 (0.112 ± 0.015) | 0.095–0.121 (0.108 ± 0.010) |
| female | 0.095–0.119 (0.104 ± 0.008) | 0.082–0.118 (0.100 ± 0.012) | 0.077–0.132 (0.096 ± 0.019) | 0.097 |
Comparisons. The following set of morphological characters clearly separates adult Phrynoglossus swanbornorum sp. nov. from all congeners: the presence of a rounded canthus rostralis, short snout (SL/SVL ratio 8.4%), rounded tongue without notch, small and indistinct tympanum, granularly textured dorsum with scattered tubercles, dorsal tubercles on flanks tipped in brownish white to white, and the absence of a lateral line, finger discs, tarsal tubercle, inner and outer palmar tubercles, fringe of skin on outer side of fifth metatarsal, and tarsal fold.
When compared against the published morphological characteristics of all other described species of Phrynoglossus (also compiled in Appendix), the new species is most morphologically similar to the P. martensii complex and differs from its congeners as follows (condition for P. swanbornorum sp. nov. in parentheses):
Phrynoglossus baluensis exhibits an inverted U-shaped ridge on the dorsum (absent), has paired vocal sacs (singular), relative finger lengths of I ≤II (II<1), has an outer metatarsal tubercle (absent), and its venter is rugose with brown blotches (smooth, uniform cream white).
Occidozyga berbeza is a smaller frog reaching 16–18 mm SVL in males and 16–19 mm SVL in females (males 23.2–28.6 mm; females 30.6 mm), exhibits a single tooth-like projection at the tip of the mandible (absent), has relative finger lengths of II<I<IV<III (IV<II<I<III), has pear shaped toe discs (absent), has a fringe on skin of metatarsals (absent), and exhibits a tarsal fold (absent).
Phrynoglossus celebensis lacks a canthus rostralis (rounded), has nostrils equidistant between eyes and tip of snout (closer to tip of snout than eyes), has vertically oriented eyes (laterally), has finger discs (absent), and fully webbed toes (moderately webbed).
Phrynoglossus diminutivus is a smaller frog reaching 18.6 mm SVL in males and 26.4 mm SVL in females (males 23.2–28.6 mm; females 30.6 mm), exhibits a single tooth-like projection at the tip of the mandible (absent), has paired vocal sacks (singular), and has a fringe on skin of metatarsals (absent).
Phrynoglossus floresianus is a larger frog reaching 52.6 mm SVL (males 23.2–28.6 mm; females 30.6 mm), lacks a canthus rostralis (rounded), has nostrils equidistant between eyes and tip of snout (closer to tip of snout than eyes), exhibits fingertips with large discs (absent), has relative finger lengths of II<I<IV (IV<II<I), has fully webbed toes (moderate), and exhibits large toe discs (absent).
Phrynoglossus laevis is a larger frog reaching 21–37.4 mm SVL in males and 31.6–48 mm SVL in females (males 23.2–28.6 mm; females 30.6 mm), lacks a canthus rostralis (rounded), has vertically oriented eyes (laterally), has finger discs (absent), two palmar tubercles (one), toe tips with disc (absent), a fringe on skin of metatarsals (absent), a tarsal fold (absent), and retains a lateral line (absent).
Occidozyga lima is a larger frog reaching 21–37.4 mm SVL in males and 31.6-48 mm SVL in females (males 23.2–28.6 mm; females 30.6 mm), lacks a canthus rostralis (rounded), exhibits a single tooth-like projection at the tip of the mandible (absent), has a tongue that is pointed behind (rounded), vertically oriented eyes (laterally), nostrils that are equidistant between eyes and tip of snout (closer to tip of snout than eyes), an indistinct supratympanic fold (distinct), lacks distal subarticular tubercles on fingers III and IV (present), has toe tips with discs (absent), an outer metatarsal and a tarsal tubercle (absent), a tarsal fold (absent), retains lateral line system (absent), and has a venter covered in pearly tubercles (smooth).
Phrynoglossus magnapustulosus is a smaller frog reaching 17–20 mm SVL in males and 14–21.3 mm SVL in females (males 23.2–28.6 mm; females 30.6 mm), lacks a canthus rostralis (rounded), has two inner palmar tubercles (single), a fringe on skin of metatarsals (absent), and a tarsal fold (absent).
Phrynoglossus martensii females reach up to 45 mm SVL (30.6 mm), lacks a canthus rostralis (rounded), has an interorbital distance wider than the internarial distance (interorbital region smaller than upper eyelid), an interorbital distance about three times wider than upper eyelid (interorbital region smaller than internarial distance), has relative finger lengths of II =IV (IV<II), distal subarticular tubercles on fingers III and IV (absent), relative toe lengths of I<II<III<V<IV (I<V<II<III<IV), a fringe of skin on outer side of fifth metatarsal (absent), and a tarsal fold (absent).
Phrynoglossus myanhessei has an interorbital distance greater than width of upper eyelid (interorbital distance smaller than upper eyelid), internasal distance less than interorbital distance (internasal distance greater than interorbital distance), relative finger lengths of II<IV<I<III (IV<II<I<III), palmar tubercles are bifid (singular), relative toe lengths of I<II<V<II<IV (I<V<II<III<IV), has toes that are almost fully webbed (moderately webbed), an outer metatarsal tubercle (absent), a fringe on skin of metatarsals (absent), a tarsal fold (absent), and the dorsolateral stripe is absent (sometimes present).
Phrynoglossus semipalmatus females reach up to 35–48 mm SVL (30.6 mm), lacks a canthus rostralis (rounded), exhibits a single tooth-like projection at the tip of the mandible (absent), has nostrils equidistant between eyes and tip of snout (closer to tip of snout than eyes), fingertips with discs (absent), relative finger lengths I<II =IV (IV<II<I<III), toe tips with discs (absent), feeble fringe of skin on metatarsals (absent), feeble tarsal fold (absent).
Phrynoglossus sumatranus females reach 35–61.6 mm SVL (30.6 mm), exhibits a single tooth-like projection at the tip of the mandible (absent), has vertically oriented eyes, (laterally), an interorbital region smaller than width of the eyelid (interorbital region smaller than the width of upper eyelid), an indistinct supratympanic fold (distinct), relative finger lengths of III<IV (IV<III), toes that are fully webbed to discs (moderately webbed), toe tips with discs (absent), a fringe of skin on metatarsals (absent), retains a weak lateral line system (absent), has a blackish gular (brown with white mottling), and a dark brown band on either side of the cloaca (absent).
Phrynoglossus tompotika has a snout equal to diameter of eye (snout smaller than diameter of eye), a loreal region that is concave (convex), exhibits a single tooth-like projection at the tip of the mandible (absent), paired vocal sac (singular), fingers with discs (absent), relative finger lengths of II<I<IV<III (IV<II<I<III), two inner palmar tubercles (singular), relative toe lengths of I<II<V<III<IV (I<V<II<III<IV), toes with discs (absent), toe discs with circummarginal groove (absent), and a tarsal fold (absent).
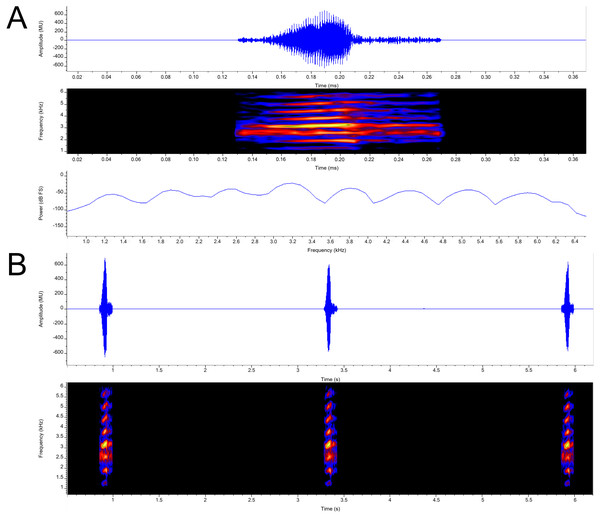
Call (Fig. 7). Each call is a high-pitched “yip” that consists of single pulsatile note. Call duration measured 0.102 –0.125 s ( = 0.114 ± 0.009; n = 7) with an intercall duration of 2.315–3.064 s ( = 2.628 ± 0.264; n = 6). Call period measured 2.426−3.168 s ( =2.741 ±0.264; n = 6). Call repetition rate measured 0.316 –0.412/s ( = 0.365 ± 0.034; n = 6). The calls exhibit narrowly separated parallel frequency bands. Calls contain a loosely defined central pulse group (28–31 pulses per call; = 30.143 ± 1.574; n = 7) with indistinguishable amplitude modulation present on either end of the call. Pulse group duration measured 0.045–0.052 s ( = 0.050 ±0.003; n = 7). Pulse rate varied from 593.870 − 622.490/s ( = 608.947 ± 11.716; n = 7). Apart from the pulsatile nature of calls, amplitude modulation is detected, with energy increasing and an occasional (n = 5 out of 7) slight drop of energy near the middle of the call, subsequently reaching its maximum energy within 3-5 pulses, then decreasing again. Minimum and maximum frequencies were measured at 5% and 95% respectively and ranged from 2,498-4,393 Hz with both maximum call energy and maximum frequency at 3,187 Hz; n = 7.
Figure 7: Advertisement calls from a single male P. swanbornorum sp. nov.
(A) Audio spectrogram, oscillogram, and power spectrum views of a single note (B) audio spectrogram and oscillogram views of multiple notes. Macaulay Library catalogue number ML237498.Call data of congeners have been completely described for P. sumatranus (Peters, 1877; Márquez & Eekhout, 2006), P. magnapustulosus (Taylor & Elbel, 1958);(Köhler et al., 2021), P. martensii populations in Myanmar, and P. myanhessei (Köhler et al., 2021). The call of Phrynoglossus swanbornorum sp. nov. was recorded at a higher ambient temperature (30.1 C) than that of P. sumatranus (24.5–29 C). The call descriptions for P. magnapustulosus, P. martensii, and P. myanhessei lacked data on climatic condition. The call of O. lima (Deesrisai et al., 2015) and of two disjunct populations of the P. martensii complex (Wang et al., 2016; Deesrisai et al., 2015) have been partially described but lack terminology definitions, graphic representations, and data on climatic condition. The described calls of P. martensii are from three distinct populations: population (1) Huangzhu Town, Ding’an County, Hainan Province, China (Wang et al., 2016); population (2) Khao Ang Rue Nai Wildlife Sanctuary, Chachoengsao Province, Thailand; and population (3) various sites across Myanmar. The call of P. baluensis (Boulenger, 1896) is briefly described as a series of low-pitch, raspy notes (Haas, Das & Hertwig, 2020). To compare the call of O. lima to Phrynoglossus swanbornorum sp. nov., “element” (Deesrisai et al., 2015) is equated to “call” as O. lima has a multiple note or “element” call, whereas all other species compared have a single note call.
The call of Phrynoglossus swanbornorum sp. nov. is differentiated mainly by having a higher pulse rate ( = 608.9/s in Phrynoglossus swanbornorum sp. nov.; = 143.2/s in P. sumatranus); intermediate intercall duration ( = 2.628 s in Phrynoglossus swanbornorum sp. nov., = 9.293 s in P. sumatranus; = 2.050 s in P. martensii population 1; = 3.278 s in P. martensii population 2; = 2.77−3.92 s for three individuals of P. magnapustulosus; = 0.213 s in O. lima); an intermediate call duration ( = 0.114 s in Phrynoglossus swanbornorum sp. nov.; = 0.052 s in P. martensii population 1; = 0.023 s in P. martensii population 2; = 0.037−0.046 s for five individuals of P. martensii population 3; = 0.090−0.104 s for three individuals of P. myanhessei; = 0.277−0.387 s for three individuals of P. magnapustulosus; = 0.165 s in P. sumatranus; = 0.05 s in O. lima); an intermediate dominant frequency (3,187 Hz in Phrynoglossus swanbornorum sp. nov.; 3,782 Hz in P. martensii population 1; 3,444–3,914 Hz for five individuals of P. martensii population 3; 2,467–2,839 Hz for three individuals of P. myanhessei; 3,447–3,761 Hz for three individuals of P. magnapustulosus; 2,742 Hz in P. sumatranus); higher minimum frequency (2,498 Hz in Phrynoglossus swanbornorum; 1,708–1,894 Hz in three individuals of P. magnapustulosus; 1,529–2,119 in three individuals of P. myanhessei); lower maximum frequency (3,187 Hz in Phrynoglossus swanbornorum sp. nov.; 11,419 Hz in P. martensii population 2; 3,830–4,493 Hz for five individuals of P. martensii population 3; 4,150–4,823 Hz for three individuals of P. magnapustulosus; 2,839–3,265 Hz for three individuals of P. myanhessei; 3,470 Hz in O. lima); and single note call in Phrynoglossus swanbornorum sp. nov., P. sumatranus, and P. martensii, whereas O. lima has a multiple note call.
Etymology. The specific patronym epithet “swanbornorum” is in genitive plural and refers to members of the Swanborn family, who are generous supporters of the conservation efforts of the Creative Conservation Alliance.
Distribution. Phrynoglossus swanbornorum sp. nov. is currently only confirmed to occur within the Chunati Wildlife Sanctuary (21.937533 N, 92.063010 E), Chattogram District at an elevation of ca. 33 m and Teknaf Wildlife Sanctuary (20.887020 N, 92.298954 E), Cox’s Bazar District at an elevation of ca. 26 m (Fig. 1).
Natural history
Phrynoglossus swanbornorum sp. nov. is only known to inhabit lowland, coastal, mixed-evergreen forests of southeast Bangladesh, which are bounded to the east by the mountainous Chattogram Hill Tracts. Within this habitat, Phrynoglossus swanbornorum sp. nov. has only been observed near lentic water bodies under dense forest canopy and was notably absent from surrounding agricultural fields and degraded forest. An image of P. swanbornorum sp. nov. in amplexus (see Distribution; Ahm, 2014) demonstrates that this species exhibits inguinal amplexus. Additionally, eggs from the single collected female were pigmented.
Discussion
Phylogeny
Phrynoglossus swanbornorum sp. nov. formed a distinct clade from neighbouring congeners P. martensii, P. magnapustulosa, P. myanhessei, and an undescribed species from Yangon, Myanmar. The closest known congener is the undescribed species from Myanmar, last collected in 2001, and sequenced by Mulcahy et al. (2018) which was recognized as a distinct species “Occidozyga sp. A” (MG935919, MG935914, MG935921) based on their analysis of the COI gene. Following the nomenclature of Köhler et al., 2021, the Phrynoglossus clade formed a monophyly and only two Occidozyga species are recognized—O. lima and O. berbeza. The node supporting the Occidozyga monophyly is weak with Bayesian posterior probabilities (BPP < 0.80) and the bootstrap supports (BS < 70). The addition of nuclear genetic data would likely assist in evaluation of the relationship between Phrynoglossus and Occidozyga.
Morphology
Morphological descriptions of Phrynoglossus spp. and Occidozyga spp. in the peer reviewed literature were found to be incomplete and, in some cases, incongruent. We provide a compilation of morphological characters (see Appendix) from the peer reviewed literature, supplemented by grey literature, which allows for the modification of the generic diagnosis by elucidating morphological synapomorphies.
Where character state is reported, all Phrynoglossus and Occidozyga species are found to lack vomerine teeth and have nuptial pads/spines, a bony style, a forked omosternum, pigmented eggs, a distinct or indistinct supratympanic fold, reduced to absent metacarpal webbing, moderate to extensive metatarsal webbing, elongated inner metatarsal tubercle, short arms, and small tympanum covered with skin. Most species also lack a distinct canthus rostralis with the exceptions of Phrynoglossus swanbornorum sp. nov. and P. tompotika (Iskandar, Arifin & Rachmansah, 2011). Only four species are reported to have finger discs, while toes discs are present, at least feebly, in all species. Additionally, both paired and single vocal sacs have been reported and Köhler et al. (2021) describes Phrynoglossus as having an extensive mucosome whereas O. lima has a diminished mucosome. Lastly, Phrynoglossus species have been found to reach between 15 and 61.6 mm in length with females being larger than males and P. sumatranus reaches the greatest length.
Considering these findings, multiple characters used in previous diagnoses of Phrynoglossus and Occidozyga (Taylor, 1962; Iskandar, 1998; Sailo et al., 2009; Inger et al., 2017; Poyarkov et al., 2020) are found to be insufficiently descriptive and should no longer be used. For instance, the previously used character of the length of finger I being equal or subequal to finger II applies only to P. diminutivus (Taylor, 1922), O. berbeza, and O. lima. Likewise, not all species exhibit a tuberculate dorsum, i.e., P. diminutivus exhibits a smooth dorsum and tuberculate flanks. Additionally, not all species exhibit a smooth ventrum as P. sumatranus has been reported to have a rugose ventrum (Inger, 1966). Lastly, not all species lack circummarginal grooves; P. tompotika exhibits circummarginal grooves on toe disks.
The pupils of several species were commonly described as either ovoid or diamond shaped, and both Iskandar, Arifin & Rachmansah (2011) and Köhler et al. (2021) describe these character states as diagnostic for Phrynoglossus. It is now known that both diamond and ovoid can be varying states of the same character within Phrynoglossus as the pupil of an individual Phrynoglossus swanbornorum sp. nov. was observed to change from ovoid, when dilated, to diamond shaped when constricted (Figs. 5A, 5B). Therefore, it is likely that these species exhibit horizontal pupils which resemble a diamond shape when constricted and ovoid when dilated.
Several Phrynoglossus species exhibit the paedomorphic trait of lateral line retention, though it has been previously misreported in the literature and its state is not reported for seven Phrynoglossus species. Dubois, Ohler & Biju (2001) correctly claimed that the lateral line system is retained in Occidozyga (sensu Dubois) and is absent in Phrynoglossus (sensu Dubois), and a review of the literature confirms this (see Appendix). Frost et al. (2006) incorrectly cited Dubois, Ohler & Biju (2001) stating that the lateral line system was a synapomorphy for Occidozyginae and was retained in Phrynoglossus (sensu Dubois) and absent in Occidozyga (sensu Dubois). Additionally, in species where it has been described, the presence of a lateral line system corresponds with the presence of dorsally oriented eyes and nares, whereas its absence corresponds with laterally oriented eyes and nares. Therefore we predict a lateral line system to be present in P. celebensis (Smith, 1927) and absent in O. berbeza, P. baluensis, P. diminutivus, P. floresianus (Mertens, 1927), P. magnapustulosus, P. myanhessei, P. semipalmatus (Smith, 1927), and P. tompotika.
Detailed descriptions of several other characters are also lacking in the literature for Phrynoglossus and Occidozyga. The presence or absence of several types of tubercle have not been reported for nearly half the species including nuptial pads, subarticular tubercles, tarsal tubercle, and palmar tubercles. Other characters not reported for nearly half these species include color of egg, fringe of skin on metatarsals, interorbital distance, internasal distance, pupil shape, and whether a single tooth-like process/projection is present at the tip of the mandible. Some characters are reported, yet lack sufficient clarification to compare e.g., subarticular tubercles are rarely defined as those found on the toes or the fingers and vocal sac descriptions lack specification of whether they are internal or external.
A number of incongruent descriptions were discovered in the literature and from unpublished photographs (see Appendix). Phrynoglossus baluensis was reported as lacking a canthus rostralis (Boulenger, 1896) yet also having a rounded canthus rostralis (Inger, 1966). Phrynoglossus celebensis is reported to (1) have a tongue that is not rounded (Iskandar, Arifin & Rachmansah, 2011), yet also a tongue that is rounded or feebly nicked behind (Smith, 1927; Smith, 1931) (2) fingertips with discs (Smith, 1931), yet also fingertips with conical tips (Matsui et al., 2021). Phrynoglossus floresianus is described as having a tongue that is notched behind (Iskandar, Arifin & Rachmansah, 2011), yet also rounded or feebly nicked behind (Smith, 1931; Mertens, 1927). Phrynoglossus laevis is described as having (1) toe tips with no disks (Iskandar, Arifin & Rachmansah, 2011), yet also toe tips with distinct discs (Bourret, 1942; Inger, 1954; Taylor, 1962); (2) toes rather elongate (Taylor, 1962), yet also short toes (Alcala & Brown, 1998); and (3) relative finger lengths as II<=I (Bourret, 1942; Inger, 1954), yet also I<II (Taylor, 1962). Phrynoglossus martensii was described as (1) having a head wider than its length (Taylor, 1958) yet also longer than its width (Hecht et al., 2013); (2) a supratympanic fold that is sharp and distinct (Gawor et al., 2016) yet also weakly developed (Hecht et al., 2013); and (3) tarsal fold distinct (Taylor, 1962) yet also absent (Andersson, 1942). Phrynoglossus sumatrana was reported as (1) lacking a toothlike projection on the tip of the mandible (Iskandar, 1998) yet also possessing one (Inger et al., 2017); (2) having small toe discs (Iskandar, Arifin & Rachmansah, 2011), yet also “distinct enlarged digital disc[s]” (Iskandar, 1998); (3) having smooth ventral skin (Iskandar, 1998), yet also rugose ventral skin (Inger, 1966); and (4) having a broadly rounded and indistinct canthus rostralis (Inger, 1966), yet also lacking one (Peters, 1877). While some discrepancies may be the result of inter-observer error, they might also be indicative of undescribed cryptic diversity or intraspecies variation.
Amplexus
Phrynoglossus magnapustulosus and P. myanhessei were recently described as exhibiting inguinal amplexus (Köhler et al., 2021). Coupled with the observation of inguinal amplexus in P. sumatranus (Eto & Matsui, 2012), this lead Köhler et al. (2021) to claim inguinal amplexus as an autapomorphy for Phrynoglossus. Herein, we present evidence to the contrary with observations of axillary amplexus in P. martensii (Fig. 8) and P. sumatrana (Fig. 9). Therefore, we assert that inguinal amplexus is not an autapomorphy for Phrynoglossus, though it may be a useful secondary sexual character for diagnosing cryptic species.
In addition to axillary amplexus, P. martensii has also been reported to exhibit both inguinal amplexus (Ziegler, 2000; Chan-Ard, 2003) and lumbar amplexus (Sailo et al., 2009), though we believe that the usage of the term “lumbar amplexus” here may be analogous to inguinal amplexus as Blommers-Schlösser (1981) has similarly also equated the two terms and true lumbar amplexus is a rare and primitive trait (Laurent, 1964).
Distribution
No Phrynoglossus species has been previously reported in South Asia, and the only Occidozyga species reported in South Asia is O. lima. Several publications vaguely refer to Occidozyga occurring in South Asia, specifically “Bengal,” which historically refers to much of eastern India, but contemporary usage of “Bengal” would be restricted to the state of West Bengal, India or in Bangladesh. According to Günther (1864) Oxyglossus [Occidozyga ] lima is “said to occur also in Bengal,” which was restated by Boulenger (1890) in his description of O. lima as inhabiting “Lower Bengal.” Sarkar, Biswas & Ray (1992) cites Boulenger (1890) and restates that O. lima occurs in “West Bengal” with no additional observations reported.
Figure 8: Axillary amplexus in P. martensii.
Photographed by GUEK Hock Ping (Kurt) in Selangor, Malaysia. Used with permission.Figure 9: Axillary amplexus in P. magnapustulosus.
Photographed by Chris Mattison in Borneo. Used with permission.Khan (2001) describes O. lima as commonly encountered, yet our survey efforts in the Teknaf peninsula yielded only Phrynoglossus swanbornorum sp. nov. Occidozyga lima is also reported from the Chattogram Hill Tracts in southeast Bangladesh within Kaptai National Park (Ahm, 2014; Reza & Perry, 2015). Photographic evidence is provided in Ahm (2014) of two individuals, however the individuals photographed are unequivocally not O. lima due to their laterally oriented eyes. Despite being unable to definitively identify these individuals to species level solely based on these photographs, they exhibit characteristics congruent with those of P. swanbornorum sp. nov. and are reported from within the predicted range of P. swanbornorum sp. nov. Therefore, we suspect that all reports of O. lima from southeast Bangladesh (Khan, 1997; Khan, 2001; Ahm, 2014; Khan, 2016; Reza & Perry, 2015) are instead P. swanbornorum sp. nov.
Occidozyga lima was also reported from northern Bangladesh in Nilphamari District (Kabir, Hawkeswood & Makhan, 2020), though all photographs of these observations were lost, and no specimens were taken (A. Kabir, 2020, pers. comm.). However, extensive herpetofaunal sampling efforts in northeast Bangladesh did not yield observations of O. lima (Hakim et al., 2020). Likewise, informal surveys conducted in central Bangladesh within Bhawal National Park and in northwest Bangladesh within rural areas near Rajshahi did not detect the presence of Occidozyga or Phrynoglossus (S Trageser, S Rahman, 2011-2019, SCR personal observations). Furthermore, surveys described in Khan (2001) did not observe these genera within the Sylhet region in northeast Bangladesh. No observations of Occidozyga are known between Nilphamari District and the northernmost known locality of Kaptai National Park, a distance of approximately 480 km, despite regions between these two observations having been surveyed. The observations reported by Kabir, Hawkeswood & Makhan (2020) are likely that of either an introduced population or are a misidentification of another taxon. Thus, no individuals of Occidozyga lima or Phrynoglossus spp. have been reported in Bangladesh outside of the Chattogram Division, despite surveys in the moist, deciduous sal (Shorea robusta) forest of central Bangladesh, the Sundarbans mangrove system in south-central Bangladesh, and the semi-evergreen hill forests of northeast Bangladesh (S Trageser, S Rahman, 2011-2019, SCR personal observations). Therefore, the range of the genera Occidozyga and Phrynoglossus likely does not extend to West Bengal.
We have confirmed the occurrence of P. swanbornorum in both Chunati and Teknaf Wildlife Sanctuaries and the aforementioned probable records from Kaptai National Park, 55 km to the north of Chunati Wildlife Sanctuary, would represent the furthest observation to the northwest of any Phrynoglossus spp. Additionally, Phrynoglossus swanbornorum sp. nov. appears to be the westernmost distributed species of Phrynoglossus, so this genus presumably reaches its western limit in the lowland forests of southeast Bangladesh. Considering the biogeography of the region, such a distribution is congruent with the hypothesis that the lower Ganges and Brahmaputra River act as barriers to dispersal, thereby separating many faunal lineages of South and Southeast Asian decent (Reza, 2010; Trageser et al., 2017).
Conservation and threats
We suggest this taxon be listed within Bangladesh as EN based on criteria B1ab(i,ii,iii,iv) + 2ab(i,ii,iii,iv), as it has an estimated area of occupancy (AOO) of 32 km2 and extent of occurrence (EOO) of 31,284 km2. The provided AOO and EOO include confirmed locations of Chunati and Teknaf Wildlife Sanctuaries, historical points from Myanmar, and the probable locality of Kaptai National Park. The status would not change if the assessment did not include the Myanmar specimens. Additionally, the number of subpopulations is inferred to be experiencing continuing decline due to ongoing habitat destruction, agricultural contamination, vehicular mortalities, mortality via frequent foot traffic in breeding pools within walking trails, and depredation by domestic dogs. Similar threats are known to impact P. floresianus and P. tompotika populations, which are considered Vulnerable (IUCN SSC Amphibian Specialist Group, 2019a; IUCN SSC Amphibian Specialist Group, 2019b) and Critically Endangered (IUCN SSC Amphibian Specialist Group, 2019a; IUCN SSC Amphibian Specialist Group, 2019b), respectively. Chunati and Teknaf Wildlife Sanctuaries and Kaptai National Park offer formal protection for Phrynoglossus swanbornorum sp. nov., unfortunately, these sanctuaries currently lack enforcement to adequately protect Phrynoglossus swanbornorum sp. nov. habitat. Satellite imagery analysis indicates that there are no intact forested landscapes within the known range of Phrynoglossus swanbornorum sp. nov., and sufficiently dense forested habitat is severely fragmented, including within these protected areas (Global Forest Watch, 2014). Lowland coastal forests are imperiled habitats in Bangladesh (Global Forest Watch, 2014) and the predicted rise in temperature and precipitation variability due to climate change will likely exacerbate their plight, further reducing available habitat (Shishira et al., 2020).
Conclusions
We provide morphological, molecular, and bioacoustic evidence that support the validity of the new species, Phrynoglossus swanbornorum sp. nov. This species is currently only confirmed from a relatively small area in southeastern Bangladesh and is considered to have a high risk of extinction due to conspicuous and ongoing threats to the habitat it relies on. We also review and discuss inconsistencies in the literature regarding the genera Phrynoglossus and Occidozyga, particularly that of morphological characters and provide more accurate generic diagnoses for both.
Although multiple species of Phrynoglossus and Occidozyga are generally perceived to be widespread and common, this study adds to a growing body of literature demonstrating the existence of undescribed diversity within the genus, thereby masking the true extinction risk of these species (Mulcahy et al., 2018). We suggest further research be conducted to either rectify incongruent descriptions of diagnostic characters and/or better understand intraspecific variation within the genus as well as including additional nuclear genetic data. Doing so could result in further refinement of the provided generic diagnoses. We also suggest that further surveys be conducted in the northern part of Chattogram Hill Tracts, Bangladesh e.g., Pablakhali Wildlife Sanctuary and Kassalong Reserve Forest, and throughout Rakhine State, Myanmar to better understand the distribution of Phrynoglossus swanbornorum sp. nov.
Supplemental Information
Compilation of morphological characters for all 14 species of Occidozyga and Phrynoglossus
Character states were sourced from peer reviewed literature and supplemented by grey literature.