Preference and effect of gustatory sense on sugar-feeding of fire ants
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Agricultural Science, Animal Behavior, Ecology, Entomology, Zoology
- Keywords
- Solenopsis invicta, Ethogram, Behavioural bioassay, Gustatory sense
- Copyright
- © 2021 Jaleel et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Preference and effect of gustatory sense on sugar-feeding of fire ants. PeerJ 9:e11943 https://doi.org/10.7717/peerj.11943
Abstract
Background
The red imported fire ant is one of the notorious species of ants all over the world. Sugar is one of the most important components of food and necessary for the survival of ants. Because more than 70% food of fire ants is honeydew produced by Homopteran insects such as aphids and scales.
Methodology
It is well known that beetles, flies, and honey bees can recognize the sugar taste through their legs and antennae, but in the case of fire ants, no records regarding gustatory sense were published. In the current study, considering the importance of sugar bait, we investigated the gustatory sense of the fire ant workers to sucrose via behavioral sequence and gustatory behavior. First, the feeding sequence (ethogram) of the fire ant workers on most preferred sugar (sucrose) solution was observed and categorized. Secondly, the gustatory behavior of treated fire ant workers (without flagellum and foreleg tarsi treated with HCL solution) was observed on the sucrose solution. In addition, using scanning electron microscopy (SEM) techniques, we identified the possible porous sensilla types on antenna flagellum and foreleg tarsi of fire ant workers.
Results
Based on the results of feeding sequence, foreleg tarsi of workers were the main body appendages in the detection of the sucrose droplet as compared to antennae flagellum and palps. Feeding time of treated workers with HCL solution was significantly decreased on sucrose solution as compared to those workers having no flagellum. While both types of treated workers have less feeding time in comparison to normal workers. Based on the results of feeding sequence analysis and feeding time, it is indicating that the foreleg tarsi of workers play a more important role in the detection of sucrose solution as compared to antennae flagellum. Based on the SEM results, sensilla chaetic, trichoid II, and basiconic I and II have a clear pore at their tip. This study provides a substantial basis for elucidating the gustatory function of antennal and tarsal sensilla on appendages of fire ant workers to sugars and further baits improvement for the management of fire ants.
Introduction
The red imported fire ant, also known as the fire ant or RIFA, and the worst invasive species of ants in the world, especially in China (Vinson, 1997; Wang et al., 2019; Zeng et al., 2005) and has been considered as a “super pest” because of its recorded, sometimes severe, impacts across a broad range of economic sectors, as well as on health, environment and people lifestyle (Wylie, Yang & Tsuji, 2020). Colonies of S. invicta may be monogyne or polygyne. Monogyne colonies have single queen while polygyne colonies have multiple queens. Way of reproduction and dispersion vary in these two forms of colonies (Eliyahu et al., 2011). Newly mated polygyne queens of fire ants can lay 20–30 eggs/day. While, a newly mated monogyne queen can lay 200 eggs/day, and a mature monogyne queen can lay 800–1,000 eggs/day (Ortiz-Alvarado & Rivera-Marchand, 2020). Larvae hatch from eggs after 8–10 days. The larval stage takes 6–12 days, and the pupa stage usually takes 9–16 days. Queen feeds the first brood of young larvae with oils regurgitated from her crop, trophic eggs, secretions from her salivary glands and with her broken wing muscles (Ortiz-Alvarado & Rivera-Marchand, 2020; Vinson, 1997).
The feeding behavior of S. invicta workers to granular or solid bait have been studied extensively (Howard & Tschinkel, 1980; Sorensen, Busch & Vinson, 1983), but were affected by multiple abiotic factors and biotic factors (Bockoven, Wilder & Eubanks, 2015; Vogt et al., 2003). The S. invicta workers prefer liquid food e.g., sugar solution in comparison to solid food and though previous studies indicated that liquid food was the most frequently collected material in fire ant workers (Qiu et al., 2014; Wang et al., 2018). Sugar is one of the major basic food components necessary for ant development and survival (Skinner, 1980; Tennant & Porter, 1991). Because morethan 70% food of S. invicta is honeydew produced by Homopteran insects such as aphids and scales (Blüthgen & Fiedler, 2004; Huang et al., 2018; Madsen, Sørensen & Offenberg, 2017; Skinner, 1980; Stanley, 2004; Wang et al., 2018; Wang et al., 2020).
Insects have a complex gustatory system and consists of different body appendages e.g., mouthparts, legs, palps and wings (Amrein & Thorne, 2005; Stocker, 1994; Thoma, Kobayashi & Tanimoto, 2017; Yosano et al., 2020). Feeding ethogram of insects has a specialized sequence for acceptance and intake of food (Thoma et al., 2016). Antennae and legs are useful body parts and synchronized with various function, e.g., the most important are olfaction, gustation and locomotion (Blaesing & Cruse, 2004; De Brito Sanchez et al., 2014; Dickinson et al., 2000; Malte & Holk, 2019; Mongeau et al., 2013). When hungry insects come across food, then immediately stop their movement of body appendages and performed the following behaviors. First, insects must decide if food is acceptable or not; second, indeed they have a systematic feeding sequence, and third, locomotion of body appendages slow and stop so that they can only focus on feeding (Dethier & Elizabeth, 1977; Joseph & Carlson, 2015; Thoma, Kobayashi & Tanimoto, 2017). Feeding sequence explains the main role of antennae, mouthparts, legs and ovipositor in gustatory taste (Araya & Padilla, 2020; Thoma, Kobayashi & Tanimoto, 2017). A lot of the works regarding ethogram of the feeding behavior have been reported on the model insect Drosophila, as well as on honey bees, lepidopterans, and mosquitoes (Agnihotri, Roy & Joshi, 2016; Amrein & Thorne, 2005; De Brito Sanchez et al., 2014; Thoma, Kobayashi & Tanimoto, 2017; Won Jung et al., 2015). However, the ethogram of feeding behavior in fire ants is still unknown.
In insect, ablation is the most useful technique for blocking the sensitivity of sensilla (Chieng, Hee & Wee, 2018; Kunert & Weisser, 2005; Pontes et al., 2014; Potting, Perry & Powell, 2005). The pairs of palps and/or antennae of male B. dorsalis was removed via sterile fine forceps to study the response in detection of methyl eugenol (Chieng, Hee & Wee, 2018). In honey bees, to block the sensitivity of antennae, two-silicon compounds were used to cover the antennae (Letzkus et al., 2006). HCl solution is the best acid to deactivate sensilla of antennae and legs in insects (Ramaswamy, Ma & Baker, 1987). And Ramaswamy, Ma & Baker (1987) concluded that the HCL solution blocked the sensitivity of sensilla on antennae and leg tarsi of Heliothis virescens. However, no records were published on fire ants.
Sensilla basiconic and trichoid on antennae of Drosophila, ants and H. armigera play the main role in chemoreceptors for mainly olfactory and partially gustatory sense (Ning et al., 2019; Shields et al., 2018). Several studies have been reported on the sensilla types of antennae in fire ants via scanning electron microscope (SEM) (Hurchalla & Drelich, 2019; Ning et al., 2019; Renthal et al., 2003). Six sensilla types were reported on antennae flagellum in fire ant worker e.g., sensilla basiconic, trichoid, coeloconic, coelocapitular, ampullaceal, and chaetic (Renthal et al., 2003). However, the sensilla types on legs of fire ants have not been explained.
When an insect comes in contact with the surface of the host, at first use their tarsi; therefore, tarsi are important gustatory part of appendages for host acceptance (Chun & Schoonhoven, 1973; Yosano et al., 2020). In flies and moths, foreleg tarsi have gustatory sensilla to sweet, bitter, and sour compounds (Chun & Schoonhoven, 1973; Solari et al., 2016; Yosano et al., 2020; Zhang et al., 2011). Tarsus sensilla of fruit flies, blowflies, honey bees and beetles have the gustatory sense to sugar compounds, especially sucrose solution (Chun & Schoonhoven, 1973; De Brito Sanchez et al., 2014; Solari et al., 2016; Thoma, Kobayashi & Tanimoto, 2017; Yosano et al., 2020; Zhang et al., 2011; De Brito Sanchez et al., 2014; Thoma et al., 2016; Yosano et al., 2020). However, to the best authors knowledge, the antennal and tarsal gustatory sense of fire ants to sugars compounds are still unknown. Therefore, this study first time explains the ethogram of feeding behavior of fire ant workers on the most preferred sugar solution (sucrose). We also explained the suitable techniques for blocking the sensitivity of sensilla in workers by ablation, 2-silicon components and HCL solution. Furthermore, considering the importance of antennal and tarsal gustatory sense, we observed the feeding behavior of treated fire ant workers (without antennae flagellum and foreleg tarsi treated with HCL solution) on sucrose solution. In addition, possible type of porous gustatory sensilla was speculated on antennae flagellum and foreleg tarsi of the workers.
Materials and Methods
Fire ant colonies
Colonies of fire ants were collected with soil from Nansha (22.602149 N, 113.584083 E) and Baiyun (23.394736 N, 113.737307 E) Districts, Guangzhou city, Guangdong Province, the Peoples’ Republic of China in 2019. Fire ant colonies with soil were shoveled into a bucket (20-litre capacity) and then transferred to the laboratory in the Plant Protection Research Institute, Guangdong Academy Agricultural Sciences, located in No. 7 Jinying Rd., Tianhe District 510640, Guangzhou city, Guangdong Province, China.
Colonies of fire ants were extracted from the bucket by slowly dripping water (Banks et al., 1981; Lei et al., 2019). An iron sieve (9 cm) was used to swab the floated ant raft into the plastic container (45 × 38 × 15 cm) lined with Fluon F4-1 to prevent the ants from escaping. Each nest container was fitted with the artificial nest that made in a Petri dish (15 cm diameter × 1.5 cm high) with a layer of 1.25 cm plaster. The lid of the nest was coated with black color. Water was provided in a glass test tube (2.5 × 19.5 cm), which was half-full of water and closed with cotton. A Petri dish (9 × 1.5 cm) containing minced mealworms, Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae), was placed in the plastic container and replaced twice a week. Colony populations were estimated by measuring the weight (g). We found the weight of 1,000 workers was arournd 1 g. Each colony was anesthetized with CO2 for 10 s. Than we collected the workers wih soft forcep and measured their weight. And the exact number of queens were counted by removing the lid of nest. So in our laboratoy, fire ant colonies consisted of 10,000 workers, more than 10 queens, and more broods. The controlled environment chambers were set at a photoperiod of 14:10 (L:D) h, at 25 ± 2 °C and 60–70% relative humidity. All colonies were acclimatized for 20 d in the laboratory before the experiments.
Sugar preference
In this experiment, the most preferred sugars were tested among glucose (monosaccharides), sucrose (disaccharides), raffinose (trisaccharide), cellulose (polysaccharides), and honey solutions in the laboratory. Each sugar solution was prepared at 20% concentrations with distilled water. The treatment of distilled water was used as a control. All groups of sugars were purchased from Sigma–Aldrich Co. (St. Louis, MI, USA).
Fire ant workers were anaesthetized using CO2 for the 50 s. In a multiple-choice test, about one hundred of workers of fire ants were collected by fine and soft forceps and shifted into a plastic container (15 × 11 × 9) and meanwhile deprived of any carbohydrate source and water for 48 h. The diluted Fluon solution was pasted halfway up the inner surface of each plastic box to prevent fire ant from escaping. Each sugar solution was provided using a glass test tube (10 ml), which was half-full of 20% sugar solution and closed with cotton. Meanwhile, six glass tubes (five different sugar solutions and distilled water) were placed into the plastic container. The sugar preference of workers was estimated by comparing the number of workers feeding on different sugar solutions and distilled water (Huang et al., 2018). Glass tubes were observed for 6 h to record the number of workers which fed on each solution in a day. Each glass tube was observed for 2 min per hour. The experiment was replicated six times for each colony, and a total of 4 colonies were tested.
Ethogram of the olfactory and gustatory behavior of fire ant workers on sucrose
The purpose of the experiment is to find out the behavioural sequence of workers’ detecting and deciding to feed on the preferable sugar, i.e. sucrose. An individual worker was transferred into a Petri dish (3 × 1.5 cm) by a fine and soft forceps for feeding pattern on sucrose. The worker was allowed to acclimatize the environment of the Petri-dish for at least 48 h, and then the Petri dish was placed in the dark black condition for 2 h (De Brito Sanchez et al., 2014). A droplet (10 μL) of 20% sucrose solution was dispensed by a micropipette (1–20 μL) into the centre of the Petri dish. Its foraging behaviour was observed until touching a droplet up to 20 min. If a worker would not access to the droplet, then it was discarded. Observations were done for 3 h under a Keyence VHX-5000 digital microscope (Jaleel et al., 2018; Pozuelo, Chang & Yang, 2015). The controlled conditions were similar to described above. Twenty-five workers were tested from each colony. A total of four colonies were used for categorizing olfactory and gustatory ethogram of workers.
Localization of gustatory sensilla of fire ant workers
Optimization of treatment for blocking gustatory sensilla on antennae and foreleg tarsi
The gustatory sense of workers was evaluated on antennae and foreleg tarsi after blocking the sensitivity of sensilla by ablation, 2-silicon components or HCL solution according to the modified methodologies from some previous studies (Pontes et al., 2014; Letzkus et al. 2006; Ramaswamy, Ma & Baker, 1987).
(a) Ablation
Around 1,000, fire ant workers were collected and shifted into the plastic containers as described in the section of feeding ethogram. The workers were deprived of any carbohydrate source and water for 48 h. A total of 4 colonies were used for the experiment, 30 workers from each colony. After starvation, 30 workers were separated into a glass vial (10 ml capacity with Fluon F4-1 to prevent the ants from escaping) from the sub colony. The glass vial containing workers were immobilized on the icebox. Under dissecting microscope (Carl Zeiss, Germany), a worker was fixed inside the small plastic centrifuge tube (0.2 ml) (the tip of centrifuge tube was removed) by extruding the antennae and forelegs tarsi. A sterile fine scissor (8.5 cm in length, the tip of scissor 8 mm) was used to cut antennae and forelegs tarsi.
(b) 2-silicon compounds
Workers were collected and prepared for the experiment as the same described in the section of ablation. Then 2-silicon components were mixed by ratio 1:1 and coated thinly on antennae flagellum. Similarly, forelegs tarsi were coated by silicon compounds (Huge Kouqiang Cailiao Ltd. Shandong, China).
(c) HCL treatment
HCL solution is more suitable for blocking the sensitivity of sensilla. Workers were collected and prepared in the experiment as the same described above. Five different concentrations of HCL solution (10%, 20%, 30%, 40% and 50%) were prepared and applied on the worker forelegs tarsi for the 20 s using pasture pipette under dissecting microscope in a preliminary test, in which mortality of the workers was less than 20% in the treatment of 20% HCL solution.
Two main experiments were designed as following: antennal treatments with ablation of the flagellum (Flagellum−); with 2-silicon compounds (Flagellum Silicon), without any measures (Intact), and forelegs-tarsal treatments with ablation (Tarsi−), with 2-silicon compounds covered (Tarsi Silicon), with HCL solution (Tarsi HCL), and without any measures (Intact) as control.
A treated individual worker was gently placed in the centre of a Petri-dish (3 × 1.5 cm) by a fine and soft forceps, which upper half walls were coated with Fluon solution. Movement of the treated worker was recorded for 600 s with a camera (DNT, Dietzenbach, Germany, Digi Micro 2.0 Scale, resolution: 800 pixels) centred 70 cm high over the arena floor. The arena was surrounded by white cardboard with white neon lights from above to homogenize the light and to mask any visual cues that might have influenced the worker’s behaviors.
Gustatory sense of treated fire ant workers
Ablation of the antennal flagellum and HCL treatment for forelegs tarsi were suitable and caused less effect on the movement of workers as compared to others, e.g., the 2-silicon-components for both antennae and legs and ablation of tarsi.
The treated workers were prepared as described above and designed as follows: flagellum ablation (Flagellum−), HCL-treated foreleg tarsi (Tarsi HCL), ablated flagellum and HCl-foreleg tarsi (Flagellum− + Tarsi HCL), and without any measures (Intact). The treated worker was shifted into a Petri dish (3 × 1.5 cm). The Petri dish was placed for 2 h in a dark back condition before the experiment. A droplet (10 μL) of 20% sucrose solution was dispensed using a micropipette in the centre of the dish. The foraging was not observed until to reach a droplet up to 20 min. If a worker would not be accessed to the droplet, then it was discarded. Observations were done for 3 h using a Keyence VHX-5000 digital microscope. The pre-feeding time and feeding time of each treated and intact worker were calculated. This experiment was replicated with 120 times/treated worker, the 30 workers from one colony. A total of 4 colonies were used for this experiment.
Fine location of potential gustatory sensilla of workers using a SEM
Gustatory sensilla are located at the tip of body appendages, e.g., antennae, legs, mouthparts and wings, while gustatory sensilla contained a pore at the tip (Yosano et al., 2020). We examined the antennae and forelegs tarsi of fire ant workers to clarify whether antennal and tarsal gustatory sensilla are common in the fire ants. Meanwhile, we scan the possible gustatory sensilla on forelegs tarsi and antennae. We used SEM to have a look at sensilla types on forelegs tarsi and antennae flagellum.
Flagellum and forelegs tarsi of workers were ablated with a pair of surgical scissors. Samples were fixed over 12 h, and then the samples were cleaned in phosphate-buffered saline (pH = 7.2). After this, each sample was washed in 75% ethanol for 900s. All samples were placed inside the ultrasonic wave cleaner for 30 min. The samples were dehydrated in an ascending series of ethanol washes (60, 70, 80, 90 and 100% for 20 min at each concentration). Then the samples were washed in 100% ethanol. The prepared specimens were dried for 24 h. Antennae and foreleg tarsi were fixed onto a stub and sputter-coated using an SCD 500 sputter ion instrument. The SEM (model no. HITACHI S-3400N; Japan) was used in this study (Ghaninia et al., 2018; Ramirez-Esquivel, Zeil & Narendra, 2014; Renthal et al., 2003).
Statistical analysis
All data in the bar graph were presented as mean ± SEM. Statistical tests were conducted using SPSS version 22.0 (International Business Machines Corp., Armonk, NY, USA). Feeding sequence of workers on the sucrose solution was given in the form of frequency. Velocity (cm/s), distance moved (cm), and body rotation (frequency) of the treated workers with antennae flagellum (without antennae flagellum, covered with 2-silicon compounds, and normal workers) and foreleg tarsi (with ablated forelegs tarsi, covered with 2-silicon compounds, treated with HCL solution, and normal) were calculated by automatic tracking software (EthoVision_XT 7.0; Noldus Information Technology, http://www.noldus.com). The velocity of 0.08 cm/s (mean distance moved by a worker per unit time), the distance moved (cm) (total distance travelled during the 600 s), and the body rotation as the number of clockwise and counterclockwise rotations made by the fire ant workers, in which the definition of rotation can be freely chosen (for instance turning 360°, 180°, or 90°). The data (mean ± SEM) of velocity, distance moved, and body rotation of treated workers with antennae flagellum were analyzed in the SPSS using a Kruskal–Wallis rank-sum test followed by a multiple comparison test between the stages (Siegel, 1956). Similarly, the same test was run for statistical differences among the treatments of tarsus-treated workers.
The data (Mean ± SEM) of pre-feeding and feeding time (min) treated workers without antennae flagellum (flagellum−), HCL- treated foreleg tarsi (Tarsi HCL), both Flagellum− + Tarsi HCL, and the untreated individuals (Intact) were analyzed in SPSS using statistical significance (0.05) using one-way ANOVA and followed by posthoc Tukey.
Final adjustments of brightness and contrast of SEM pictures were made using Adobe Photoshop CS6.
Results
Sugar preference
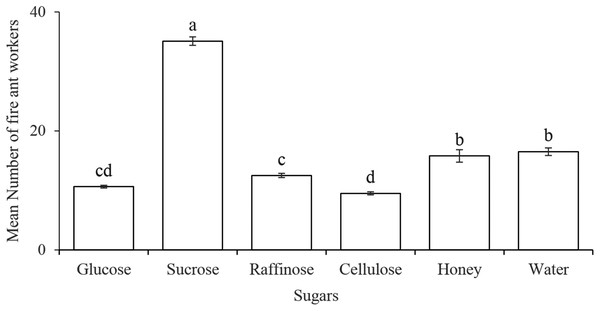
The number of the workers (35.08 ± 0.71) was significantly higher on the sucrose solution as compared to the other four categories of sugar solutions (glucose, raffinose, cellulose, and honey) and distilled water (F5,138 = 238, P < 0.0001). While no difference observed between the honey solution and distilled water (control). Among four different classes of sugars, the disaccharide of sucrose seemed more suitable as attractant to the workers (Fig. 1).
Figure 1: Preference of Solenopsis invicta workers to several sugar solutions.
Statistically significant differences among sugar solutions are denoted by different letters at P = 0.0001.Ethogram of the olfactory and gustatory behavior of fire ant workers on sucrose
The feeding sequence of the fire ant workers was classified into olfactory and gustatory phases according to their direct and indirect contact to a droplet of solution (Table 1).
| Sense mechanism | Phase | Subphase | Estimated criteria | Active workers | Stop feeding and excluded | Worker number moving to next stage |
|---|---|---|---|---|---|---|
| Olfactory phase | A. Introducing a fire ant worker | A1. No movement | 8 | |||
| A2. Approach to the sucrose droplet | Pre-feeding time of fire ant workers | 92 | 92 | |||
| Gustatory phase | B. making decision for Feeding | B. Non-feeders | B1.1 Touch with antennae, fore, mid, and hind legs | 8 | 84 | |
| B1.2 Touch with mandibles and forelegs. | 4 | 80 | ||||
| B2. Feeders | B2.1 Touch with antennae | 23 | 80 | |||
| B2.2 Touch with foreleg tarsi | 45 | |||||
| B2.3 Touch with palps | 12 | |||||
| C. Lowering Posture of Head | 80 | 80 | ||||
| D. Feeding | 80 | 80 | ||||
| E. Retraction of palps | E1. Self-grooming and stop their movement | 3 | 77 | |||
| E2. Moving back to “C” and 6-7 cycle then stop their movement | 77 | 77 |
Note:
Data were represented as frequency (n = 100).
Phase 1: Olfactory phase.
Among 100 fire ant worker, 8 workers were not moved to the sucrose droplet after introduction into the petri-dish (A1) and other fire ant workers (92) were approached to the sucrose droplet (A2) (Table 1).
Phase 2: Gustatory phase.
In A2, among 92 workers, 12 of them did not drink the sucrose droplet (B1). While, remaining 80 fire ant workers used their antennae, palps, and foreleg tarsi to touch the droplet (B2) (Table 1).
The subphase (B1) was divided into two types: the first type was those workers (8) that have no feeding on the sucrose droplet, but they used their antennae, legs, and sting to touch the droplet for 4–5 min range. After this, the workers started the grooming for 5–6 min and then stopped their movement (B1.1). While the remaining 4 workers used their mandibles and foreleg tarsi to touch the sucrose droplet. After 6–7 min, the workers pulled or dragged the sucrose droplet randomly by foreleg to the wall of Petri-dish. After this start grooming for 5–6 min and then stopped their movement (B1.2) (Table 1).
In subphase (B2), 80 workers have shown three types of response: the first type included 23 workers, and they were used their antennae to touch the sucrose droplet (B2.1). Secondly, 45 workers out of 80, were used their foreleg tarsi and palps (labial and maxillary palps) to touch the sucrose droplet with the movement of antennae-like an arm movement (B2.2). Third types included 12 workers, and they have used their palps only to touch and drink the sucrose droplet (B2.3).
All possible types of the workers in the B2 suppresses their movement of body parts (antennae and legs) and then performed as lowering posture of the head (C): in this phase, all type of feeding workers move their head down to the sucrose droplet. After this, all workers started feeding the sucrose droplet. In the feeding phase (D): fire ant workers have performed the following steps (Table 1),
-
Antennae and forelegs tarsi movement slow down and then stop.
-
Antennae became elbow-shaped.
-
Extend palps and insert into the sugar droplet and intake the sucrose droplet.
Retraction of palps (E): in this phase 3 out of 80 fed once time on the sucrose droplet After this start grooming for 5–6 min and then stopped their movement (E1). Other 77 workers were moved back to the step C–E as described above, then 7–8 cycles were completed by the workers in the E2 phase, then stop feeding and movement (Table 1).
Localization of gustatory sensilla of fire ant workers
Treatment optimization for silencing gustatory sensilla on antennae and foreleg tarsi
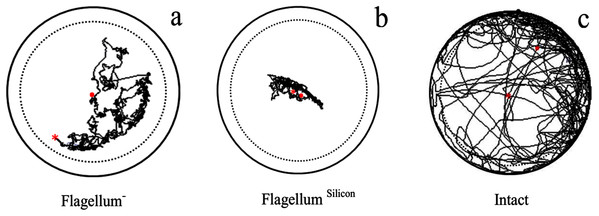
Velocity (F2,237 = 1,008, P < 0.0001), distance moved (F2,237 = 1,009, P < 0.0001), and body rotation (F2,237 = 289, P < 0.0001) of intact fire ant workers were maximum as compared to antennal treated workers (without antennae flagellum) (Table 2). The fire ant worker with ablated flagellum covered long-distance than those fire ant worker having flagellum treated with 2-silicon components (Fig. 2). The results indicated that the ablation of antennae caused less injury to workers as compared to silicon.
Figure 2: Examples of trajectory observed in antennal treated workers walking on a circular arena (Petri-dish: 3 × 1.5 cm).
With ablation of antennae flagellum as Flagellum− (A), antennae flagellum covered with 2-silicon-components as Flagellum Silicon (B), and normal worker as Intact (C). The trajectories started at the center (red × point) of the arena and last for 600 s represented as (red . point). To avoid edge effects on behavioural parameters used to describe path characteristics, delimited by the dashed line were analyzed.| Antennal treatment | Velocity (cm/s) | Distance moved (cm) | Body rotation (n) |
|---|---|---|---|
| Flagellum− | 0.38 ± 0.05 b | 474.77 ± 24.92 b | 3.16 ± 0.96 b |
| Flagellum Silicon | 0.19 ± 0.02 c | 265.76 ± 22.55 c | 2.66 ± 0.81 c |
| Intact | 1.26 ± 0.06 a | 1456.58 ± 20.24 a | 16.96 ± 1.26 a |
Note:
Flagellum−: ablation of antennae flagellum, Flagellum Silicon: antennae flagellum covered with 2-silicon compounds, and Intact: normal. n = 80 for each treated fire ant worker. Values with different letters within a column indicate significant differences among treatments for a given locomotor parameter at P < 0.05. Significant differences in locomotor parameters among treatments tested with a multiple comparison test between stages after running a Kruskal–Wallis rank-sum test. Data are given as mean ± CI0.95.
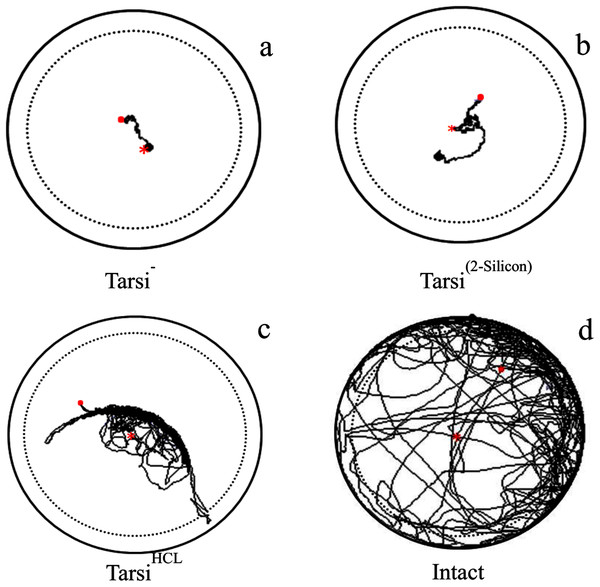
The locomotory parameters i.e. velocity (F3,316 = 1,222, P < 0.0001), distance covered (F3,316 = 1,,216, P < 0.0001), and body rotation (F3,316 = 3,426, P < 0.0001) of normal workers were maximum as compared to the tarsus treated workers i.e. workers without foreleg tarsi, foreleg tarsi covered with 2-silicon compound and foreleg tarsi treated with HCL solution (Table 3). The velocity and body rotation of fire ant worker without foreleg tarsi, and silicon treated tarsi workers were similar but less than HCL treated tarsi workers. The HCL treated tarsi workers were covered long distance than those of other two treated fire ant workers (Fig. 3). The results indicated that the HCL solution caused less injury to fire ant workers as compared to ablation and a silicon compound.
Figure 3: Examples of trajectory observed tarsi treated workers walking on a circular arena (Petri-dish: 3 × 1.5 cm).
With ablation of forelegs tarsi as Tarsi− (A), covered with 2-silicon compounds as Tarsi Silicon (B), treated with 20% HCL solution as Tarsi HCL (C), and normal worker as intact (D). The trajectories started at the centre (red × point) of the arena and last for 600 s represented as (red . point). To avoid edge effects on behavioural parameters used to describe path characteristics, delimited by the dashed line were analyzed.| Treatment | Velocity (cm/s) | Distance moved (cm) | Body rotation (n) |
|---|---|---|---|
| Tarsi˗ | 0.07 ± 0.02 c | 110.92 ± 17.53 d | 0.00 ± 0.00 c |
| Tarsi Silicon | 0.10 ± 0.02 c | 144.66 ± 14.30 c | 0.00 ±0.00 c |
| Tarsi HCL | 0.35 ± 0.02 b | 434.10 ± 28.39 b | 1.40 ± 0.49 b |
| Intact | 1.24 ± 0.06 a | 1457.58 ± 20.20 a | 16.96 ± 1.16 a |
Note:
Tarsi− ablation of foreleg tarsi, Tarsi Silicon: forelegs tarsi covered with 2-silicon components, Tarsi HCL: forelegs tarsi treated with 20% HCL solution, and Intact: normal. (n = 80 for each treatment) Values with different letters within a column indicate significant differences among treatments for a given locomotor parameter at P < 0.05. Significant differences in locomotor parameters among treatments were tested with a multiple comparison test between stages after running a Kruskal–Wallis rank-sum test. Data are given as mean ± CI0.95.
Gustatory sense of the treated fire ant workers
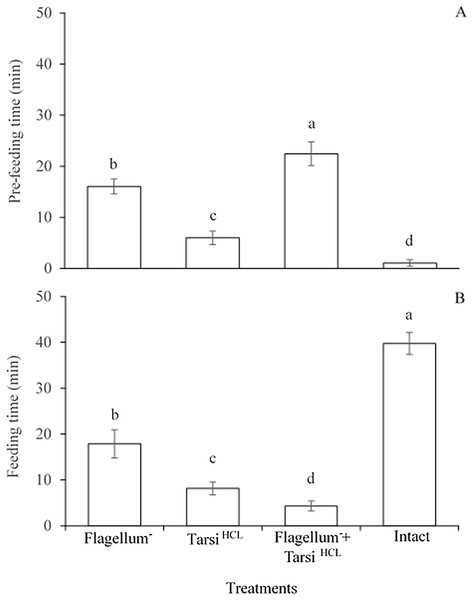
The significant difference was observed in the pre-feeding time among all treated workers e.g., worker without flagellum, the worker tarsi treated with HCL solution, and both of them (F3,476 = 1,114, P < 0.0001). While, the worker’s foreleg tarsi treated with HCL solution spent less time for searching sucrose solution as compared to other two treatments e.g., worker without flagellum and both without flagellum plus worker’s foreleg tarsi treated with HCL solution (Fig. 4A). The results implied that antennae might be more responsible for detecting sucrose droplet than foreleg tarsi during the olfactory phase.
Figure 4: Pre-feeding time (A) and feeding time (B) of the different treated workers on sucrose solution.
Within the different treated workers, the means with different letters are significantly different (One-way ANOVA, at P < 0.05) (n = 120 per treatment). Ablated flagellum (Flagellum−), HCL- treated foreleg tarsi (Tarsi HCL), Both of ablated flagellum and HCL- treated foreleg tarsi (Flagellum− + Tarsi HCL), and Untreated workers (Intact).The significant difference was observed in the feeding time among all treated workers e.g., worker without flagellum, the worker tarsi treated with HCL solution, and both of them (F3,476 = 2,352, P < 0.0001). All treated workers spent less time on feeding 20% sucrose solution in comparison to untreated/normal workers. And, in treated workers, the workers with without flagellum plus foreleg tarsi treated with HCL solution spent less time on 20% sucrose solution than those of other two treated workers (Fig. 4B). The results implied that foreleg tarsi were more responsible for tasting sucrose droplets than antennae during the feeding time. In summary, the consequences showed that the gustatory taste of fire ants for sucrose might be localized mainly on foreleg tarsi and partially on antennae.
Further location of potential gustatory sensilla of workers using a SEM
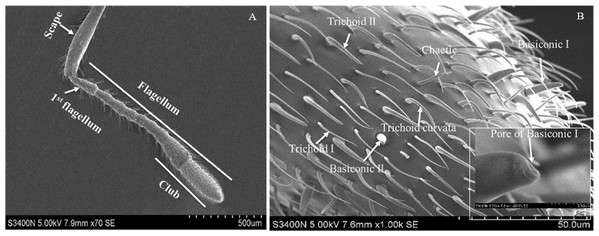
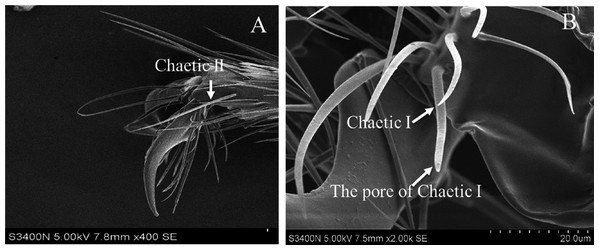
Based on the results of feeding sequence, foreleg tarsi and antennae flagellum of fire ant workers are necessary appendages and kept porous sensillae. The SEM pictures of antennal and tasus sensilla was compared and labelled by following the previous study of Ramirez-Esquivel, Zeil & Narendra (2014) and Renthal et al. (2003). Results showed that there were three major kinds of possibe gustatory sensilla in fire ant workers, i.e., sensilla trichoid I, II and curvata, sensilla basiconic I and II, sensilla chaetic on the flagellum (Fig. 5) and only one type, e.g., sensilla chaetic I and II on the foreleg tarsi (Fig. 6) of the fire ant worker.
Figure 5: Scanning electron micrographs of the fire ant workers’ antennae flagellum.
(A) Parts of fire ant workers’ antennae, (B) sensilla trichoid (Tr) I, II and curvata, sensilla basiconic (Ba) I, II, sensilla chaetic (Ch), and sensilla ampullaceal (am).Figure 6: Scanning electron micrographs of the fire ant workers’ forelegs tarsi.
(A) Sensilla Chaetic II, (B) chaetic sensilla (chaetic I) on a tarsus of foreleg near the claw, showing a sensillum pore.Discussion
Several insects use their tarsal sensilla for tasting the sugar compounds (Chun & Schoonhoven, 1973; Solari et al., 2016; Yosano et al., 2020; Zhang et al., 2011). However, in the case of fire ants, no records regarding gustatory sense were published. Therefore, the ethogram of feeding and gustatory behavior should be elucidated in fire ants.
A sucrose solution is often considered as a phagostimulant for ants in comparison to solid food (Deby, CassillWalter & Tschinkel, 1999; Madsen, Sørensen & Offenberg, 2017; O’Brien, 2005; Skinner, 1980). Our results were similar to O’Brien (2005) study that 20% sucrose solution was the most preferrable feed, and the forelegs tarsi of fire ants played the main role than antennae for detection of sucrose solution. Madsen, Sørensen & Offenberg (2017) explained that the black garden ant, Lasius niger preferred 20% disaccharides solution in comparison to 20% monosaccharides solution (O’Brien & Hooper-Bùi, 2005). The less-dense sucrose solution provides more energy to ants as compared to more viscous sucrose solution. And more viscous sugar solutions usually less preferred by ants (Detrain & Prieur, 2014). In the current study, the disaccharides (sucrose) solution was more preferred among other solutions by the workers.
Feeding sequence is an important phenomenon and plays a critical role in the gustatory behavior of insects (Thoma, Kobayashi & Tanimoto, 2017). The ethogram of feeding behavior of the model insect, Drosophila melanogaster have been studied on sugars (Scott, 2018; Joseph & Carlson, 2015; Thoma, Kobayashi & Tanimoto, 2017). Generally in insects, the feeding sequence was comprised on three principles as follows ① making appetitive exploration using body appendages, i.e., antennae, wings, and legs, ② using body appendages to contact the food and then start feeding, and ③ suppressing the locomotion of body appendage (Dethier & Elizabeth, 1977; Joseph & Carlson, 2015; O’Brien, 2005; Thoma, Kobayashi & Tanimoto, 2017). O’Brien (2005) explaned that ant becomes moion less when feed on 20% sucrose solution, similarly in our study we explained that movemenst of antennae and tarsi stopped. Both studies of O’Brien (2005) explained the liquid ingesting behaviors of individual fire ant worker. Drosophila flies and blowflies have a similar feeding sequence on a sucrose solution, and both flies recognized sugar taste through proboscis and legs tarsi (Scott, 2018; Stoffolano & John, 2019). In our study, maximum fire ant workers recognized the sucrose solution through foreleg tarsi in comparison to antennae flagellum.
Ants movements becomes slowdown when move on waxy layers or when they become injured from conspecifics over food, self-inflicted, and partial predation (Bowerman, Johnson & Bowerman, 2010; Gilad et al., 2021). The first two pairs of walking legs and antennae have additional roles in spatial chemical orientation in insects (Devine & Atema, 1982). The Myrmarachne formicaria have interesting locomotory behavior as antennal illusion behaviour where the forelegs elevated similar to their antennae (Shamble et al., 2017). A desensitve or inacive antenna makes the fly incapable of walking or climbing against gravity (Madabattula et al., 2015). Injury of antennae and legs via ablation or chemical have followings effects i.e., loss of hemolymph, suboptimal movement, and lower capacity to carry food (Gilad et al., 2021; Krause et al., 2017). The speed and movement of mutant house fly larvae was decreased when treated with low concentration of octopamine (Saraswati et al., 2010). While, the locomotor activity in the blood-sucking bug increased when treated with low concentration of eugenol (Moretti et al., 2017). The volatile component phentolamine hydrochloride prohibit locomotor activity of the immature Triatoma infestans (Reynoso et al., 2020). While in our study, ablation, HCL, and two silicon compounds decrease walking abilities of fire ant workers.
Gustatory sensilla are mostly located on the distal part of the antennae, on the tarsi of the forelegs, and on the mouthparts (De Brito Sanchez, 2011). Ablation is good techniques to block the sensitivity of sensilla in insects (Lockey & Willis, 2015; Pontes et al., 2014). Pontes et al. (2014) have been reported that ablation of antennae flagellum and forelegs tarsi were a suitable technique for the study of gustatory behavior in R. prolixus. Chieng, Hee & Wee (2018) explained the role of antennae and palps in detection of methyl eugenol, they removed both pairs of palps and/or antennae of male B. dorsalis via sterile fine forceps. They reported that neither the ablation of maxillary pulp nor antenna was found to affect the survival of the experimental flies compared to untreated, intact males. Similarly, in our experiment ablation, silicon and HCL treatement did not affect the survival of fire ant workers. The sensitivity of sensilla has been blocked successfully for study the gustatory behavior of honey bees (Letzkus et al., 2006), H. virescens (Ramaswamy, Ma & Baker, 1987), and cockroaches (Lockey & Willis, 2015). In our study, the ablation for antennae flagellum and HCL solution for foreleg tarsi were suitable techniques for blocking the sensitivity of sensilla in fire ant workers. The feeding ability of ablated beetle, R. prolixus were significantly decreased on the bitter compound (quinine) in comparison to normal beetles (Pontes et al., 2014). The ablated cockroaches were unable to approach to food in comparison to normal cockroaches (Lockey & Willis, 2015). The oviposition preference of HCL treated H. virescens was decreased on cotton and cherry in comparison to normal. In our findings, the feeding ability of flagellum ablated and HCL treated workers were significantly decreased on sucrose solution.
Gustatory sensilla of insects are uniparous and bristle like structures with the pore located at the tip (Yosano et al., 2020; Zacharuk, 1980) using Scanning electron microscopy. Antennal sensilla of Drosophila, ants, and Heliothis armigera, e.g. basiconic and trichoid have been reported as chemoreceptors (main olfactory sense and partially gustatory sense) (Chieng, Hee & Wee, 2018; Ghaninia et al., 2018; Kumar et al., 2015; Shields et al., 2018). Antennal sensilla chaetica have potential role in olfaction and gustation, in host location of female Ectropis obliqua moths (Long et al., 2018). Tarsal sensilla (chaetic) of Tribolium castaneum (Coleoptera: Tenebrionidae) have the gustatory sense to sweet, bitter, and leaf surface wax (Seada & Hamza, 2018; Yosano et al., 2020). Honey bees and blowflies can recognize the sucrose solution through their claws of the foreleg (De Brito Sanchez et al., 2014; Stoffolano & John, 2019). However, the types of antennal sensilla have been reported in the fire ants (Renthal et al., 2003). We have explored the possible porous gustatory sensilla of antennae and foreleg tarsi sensilla, e.g. basiconic, trichoid, and a chaetic were on the flagellum. While only sensilla chaetic was on the foreleg tarsi. Sensilla chaetic and basiconic have clear pore at their tip. In addition, sensilla chaetic have been reported on the antennae of four species of ants, Aphaenogaster rudis Emery, Lasius alienus Foerster, Formica subsericea Say, and Camponotus ferrigineus (F.) and supposed to be contact chemoreceptors. Based on our findings, we assumed that sensilla chaetic and basiconic on foreleg tarsi and antennae flagellum respectively might be major potential gustatory sensilla in the fire ant workers. And this study will be good reference on future work regarding the specific function of gustatory sensilla located on antennae and legs. Our findings might be a good basis for the study regarding sugar taste recognition and sugar bait improvement in the fire ant.