Brodifacoum does not modulate human cannabinoid receptor-mediated hyperpolarization of AtT20 cells or inhibition of adenylyl cyclase in HEK 293 cells
- Published
- Accepted
- Received
- Academic Editor
- Jörg Oehlmann
- Subject Areas
- Biochemistry, Cell Biology, Toxicology, Pharmacology
- Keywords
- Synthetic cannabinoid, Superwarfarin, Overdose, Cannabinoid receptor signaling
- Copyright
- © 2019 Sachdev et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Brodifacoum does not modulate human cannabinoid receptor-mediated hyperpolarization of AtT20 cells or inhibition of adenylyl cyclase in HEK 293 cells. PeerJ 7:e7733 https://doi.org/10.7717/peerj.7733
Abstract
Background
Synthetic cannabinoids are a commonly used class of recreational drugs that can have significant adverse effects. There have been sporadic reports of co-consumption of illicit drugs with rodenticides such as warfarin and brodifacoum (BFC) over the past 20 years but recently, hundreds of people have been reported to have been poisoned with a mixture of synthetic cannabinoids and BFC. We have sought to establish whether BFC directly affects cannabinoid receptors, or their activation by the synthetic cannabinoid CP55940 or the phytocannabinoid Δ9-tetrahydrocannabinol (Δ9-THC).
Methods
The effects of BFC on the hyperpolarization of wild type AtT20 cells, or AtT20 cells stably expressing human CB1- or CB2- receptors, were studied using a fluorescent assay of membrane potential. The effect of BFC on CB1- and CB2-mediated inhibition of forskolin-stimulated adenylyl cyclase (AC) activation was measured using a BRET assay of cAMP levels in HEK 293 cells stably expressing human CB1 or CB2.
Results
BFC did not activate CB1 or CB2 receptors, or affect the hyperpolarization of wild type AtT20 cells produced by somatostatin. BFC (1 µM) did not affect the hyperpolarization of AtT20-CB1 or AtT20-CB2 cells produced by CP55940 or Δ9-THC. BFC (1 µM) did not affect the inhibition of forskolin-stimulated AC activity by CP55940 in HEK 293 cells expressing CB1 or CB2. BFC (1 µM) also failed to affect the desensitization of CB1 and CB2 signaling produced by prolonged (30 min) application of CP55940 or Δ9-THC to AtT20 cells.
Discussion
BFC is not a cannabinoid receptor agonist, and appeared not to affect cannabinoid receptor activation. Our data suggests there is no pharmacodynamic rationale for mixing BFC with synthetic cannabinoids; however, it does not speak to whether BFC may affect synthetic cannabinoid metabolism or biodistribution. The reasons underlying the mixing of BFC with synthetic cannabinoids are unknown, and it remains to be established whether the “contamination” was deliberate or accidental. However, the consequences for people who ingested the mixture were often serious, and sometimes fatal, but this seems unlikely to be due to BFC action at cannabinoid receptors.
Introduction
Brodifacoum (BFC) is an inhibitor of vitamin K epoxide reductase and active ingredient of rodenticides (King & Tran, 2015). There have been sporadic reports of brodifacoum consumption with drugs such as cocaine and cannabis (La Rosa, Clarke & Lefkowitz, 1997; Waien, Hayes Jr & Leonardo, 2001; Spahr, Maul & Rodgers, 2007), however, a large number of people were recently hospitalized with poisoning by brodifacoum and related compounds following ingestion of what are believed to be synthetic cannabinoid receptor agonists (SCRAs) (Kelkar et al., 2018; Riley et al., 2019; Moritz et al., 2018; Panigrahi, Jones & Rowe, 2018). There is limited evidence to suggest that people have on occasions deliberately combined brodifacoum with cannabis (La Rosa, Clarke & Lefkowitz, 1997; Spahr, Maul & Rodgers, 2007), and the apparent mixing of brodifacoum with a variety of different SCRA could be a deliberate attempt to enhance the effects of the drugs through either a pharmacokinetic or pharmacodynamic mechanism. In this study, we have examined the effects of brodifacoum on the acute signalling of human CB1 and CB2 receptors in AtT20 and HEK 293 cells. In AtT20 cells, activation of heterologously expressed CB1 or CB2 produces a hyperpolarization, mediated by activation of G protein-gated inwardly rectifying K channels (Mackie et al., 1995; Banister et al., 2016). In CB1- or CB2-expressing HEK 293 cells, we measured the real time modulation of forskolin-stimulated cAMP accumulation (Cawston et al., 2013). We found that cannabinoid-induced signaling was not affected by brodifacoum, indicating that combining SCRA with brodifacoum is not likely to enhance user experience through interactions with cannabinoid receptors.
Methods
Drugs
(-) CP 55940 was from Cayman Chemical (#90084; Ann Arbor MI, USA), Δ9-tetrahydrocannabinol (THC) was from THCPharm (Frankfurt, Germany) and was a kind gift from the Lambert Initiative for Cannabis Therapeutics (University of Sydney). Brodifacoum was from Sigma-Aldrich (#46036), and forskolin was from Ascent Scientific Ltd.
Hyperpolarization assay
Experiments on AtT20FlpIn cells stably transfected with human CB1 (AtT20-CB1) or CB2 (AtT20-CB2) were carried out essentially as described in Banister et al. (2016). The AtT20FlpIn cells were created in our laboratory from wild type AtT20 cells we purchased from the American Type Culture Collection (ATCC CRL-1795). The assay method is based on that outlined in detail in Knapman et al. (2013). Cells were grown in DMEM (#D6429; Sigma-Aldrich, Castle Hill, NSW) supplemented with 10% fetal bovine serum (FBS, #12003C; SAFC Biosciences, Brooklyn, Victoria, Australia), 100 units penicillin/100 µg ml−1 streptomycin (1%, #15140122; Life Technologies, Scoresby, Victoria, Australia), hygromycin gold (80 µg ml−1, #ant-hg; Invivogen, San Diego, CA). Cells were grown in 75 cm2 flasks and passaged when 80–90% confluent. On the evening before experiments, cells were detached using trypsin/EDTA solution (#T3924; Sigma-Aldrich), resuspended in L-15 media (#11415064; Life Technologies) supplemented with 1% FBS, penicillin/streptomycin, and glucose (15 mM, SIGMA #G7021) and plated onto 96 well black walled, clear bottomed, culture plates which had been previously coated with poly-D-lysine (SIGMA #P6407). Cells were incubated overnight at 37 °C in a humidified incubator in room air.
Proprietary FLIPR membrane potential dye (blue, #R8034, Molecular Devices, Sunnyvale CA) was dissolved in Hank’s Balanced Salt Solution (HBSS) of composition (mM) NaCl 145, HEPES 22, Na2HPO4 0.338, NaHCO3 4.17, KH2PO4 0.441, MgSO4 0.407, MgCl2 0.493, CaCl2 1.26, glucose 5.56 (pH 7.4, osmolarity 315 ± 15) and added to the cells an hour before fluorescence reading began. Dye was used at 50% of the manufacturers recommended concentration, and cells were incubated at 37 °C in humidified room air for loading. Plates were read using a Flexstation 3 (Molecular Devices) plate reader at 37 °C. Plates were excited at a wavelength of 530 nm, emission was measured at 565 nm, with cut-off filter at 550 nm. Drugs were added using the pipetting function of the Flexstation in a volume of 20 µl after recording 60–120 s of baseline fluorescence. Readings were made every 2 s. Drug stocks were made up in DMSO (#D8418, Sigma-Aldrich) and diluted on the day of experiment, the final concentration of DMSO in the assay was 0.1%.
Data were expressed as the percentage change in baseline fluorescence produced by drug addition. The change in fluorescence produced by vehicle (0.1% DMSO) addition was subtracted from the traces before this calculation. Data is expressed as the mean ± SEM of at least 5 independent determinations performed in duplicate, unless otherwise noted. Pooled data was fit to a four-parameter logistic equation in Graphpad PRISM 7 (GraphPad Software, San Diego CA, USA).
Assay of cAMP levels
Human embryonic kidney (HEK) 293 FlpIn cells stably transfected with human CB1 or CB2 receptors tagged with three haemagglutinin epitopes at the amino terminus and human G protein gated inwardly rectifying potassium channel 4 (GIRK4) were used (the construction of these cells will be described in another place, and we did not assay CB receptor coupling to GIRK4 in this study). Cells were grown in DMEM containing 10% FBS and 100 units/ml/penicillin, 100 µg/ml streptomycin and were maintained under selection with hygromycin (80 µg ml−1) and G418 (400 µg ml−1). HEK 293 FlpIn cells were originally obtained from Life Technologies (now Thermofisher, #75007).
Cellular cAMP levels were measured using the pcDNA3L-His-CAMYEL plasmid, which encodes the cAMP sensor YFP-Epac-RLuc (CAMYEL), (Cawston et al., 2013). The pcDNA3L-His-CAMYEL was a kind gift from Dr. Angela Finch (The University of New South Wales, NSW, Australia), and originally obtained from American Type Culture Collection (Manassas, VI, USA). Cells were seeded in 10 cm dishes at a density of 6,000,0000 such that they would be 60–70% confluent the next day. The day after seeding, pcDNA3L-His-CAMYEL plasmid was transiently transfected into cells using linear polyethyleneimine (PEI, m.w. 25 kDa) (#23966, Polysciences, Warrington, PA, USA). The DNA-PEI complex mixture was added to the cells at the ratio of 1:6, and incubated for 24 h in 5% CO2 at 37 °C. After the incubation, cells were detached from the dish using trypsin/EDTA and the pellet was resuspended in 10 ml Leibovitz’s L-15, no phenol red (#21083027; Gibco) media supplemented with 1% FBS, 100 units/ml/penicillin, 100 µg/ml streptomycin and 15 mM glucose. The cells were seeded at a density of 100,000 cells per well in poly D-lysine (Sigma-Aldrich) coated, white wall, clear bottom 96 well microplates. Cells were incubated overnight at 37 °C in ambient CO2.
On the following day, drugs were prepared in HBSS containing 0.1 mg ml−1 BSA. For measurement of cAMP inhibition, all the drugs were made in 3 µM of forskolin. Coelenterazine-h substrate (2.5 µM) (#S2011; Promega, Madison, WI, USA) was added to the cells, and incubated for 5 mins prior to the addition of drugs or vehicle. Luminescence was measured using a Flexstation 3 (Molecular Devices) microplate reader at 37 °C at an emission wavelength of 461 nm and 542 nm simultaneously, with an integration time of 1 s. Drugs were added in a volume of 10 µl (10×) to each well to give the desired concentration. The final concentration of DMSO in each well was always 0.1%. Raw data are presented as inverse BRET ratio of emission at 461/542. Background reading (no substrate) was subtracted from raw values before calculating ratios. For convenience, values are expressed such that an increase in ratio correlates with increase in cAMP production. Area under the curve (AUC) analysis was performed in GraphPad prism (Graph Pad Software Inc., San Diego, CA, USA), and data were expressed as percentage of the difference between basal (vehicle, 0%) and forskolin (100%) values over a 5-minute period after forskolin addition.
For experiments examining the potential interaction between brodifacoum and cannabinoids, the cells were pre-treated with 1 µM of brodifacoum (or vehicle) and the response to a subsequent addition of SCRAs was measured. The concentration of DMSO (0.1%) was kept constant for the brodifacoum-treated and control cells. Data was normally distributed (D’Agostino and Pearson normality test, PRISM), differences between groups were tested using unpaired Student’s t-Test (PRISM). Statistical significance was defined as P < 0.05.
Results
Acute application of brodifacoum for 5 min at concentrations up to 30 µM did not significantly affect the fluorescence of AtT20 cells expressing CB1 or CB2 receptors (Fig. 1). Prolonged exposure to brodifacoum at concentrations greater than 10 µM produced decreases in fluorescence in AtT20 cells expressing CB receptors as well as wild type cells, and so for experiments examining the potential interaction between brodifacoum and cannabinoids we used a concentration of 1 µM.
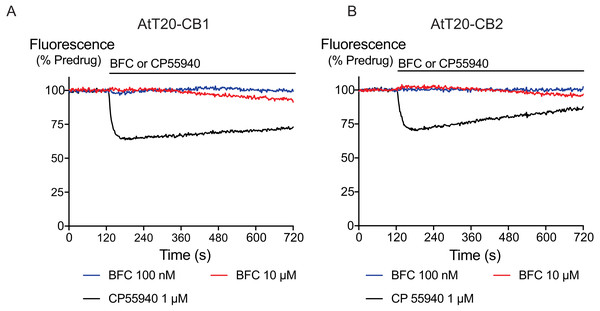
Figure 1: The effects of brodifacoum (BFC) and CP55940 in AtT20 cell expressing CB1 or CB2.
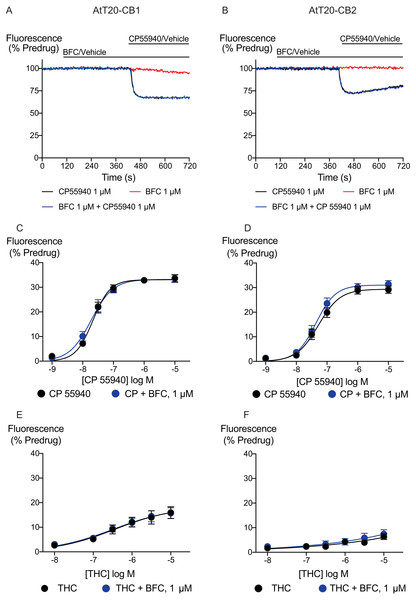
Representative traces showing the change in fluorescence induced by application of CP55940 (1 µM) but not BFC (10 µM) in (A) AtT20-CB1 and (B) AtT20-CB2 cells. Values are expressed as a percentage of predrug baseline. A reduction in fluorescence indicates a hyperpolarization. The prolonged application of BFC (10 µM) produces small changes in the fluorescence in AtT20 cells expressing cannabinoid receptors. Drug was added for the duration of the bar; the traces are representative of at least five independent experiments.We generated concentration–response curves for the high efficacy cannabinoid agonist CP55940 and the lower efficacy agonist THC after 5 min of exposure to brodifacoum (Fig. 2). In AtT20-CB1 cells, application of CP55940 produced a maximum change in fluorescence of 33 ± 1%, with a pEC50 of 7.7 ± 0.04; with the addition of brodifacoum the maximum change in fluorescence was 33 ± 1%, with a pEC50 of 7.7 ± 0.06 (P = 0.97). In AtT20-CB2 cells, application of CP55940 produced a maximum change in fluorescence of 29 ± 1.1%, with a pEC50 of 7.3 ± 0.1; with the addition of brodifacoum the maximum change in fluorescence was 31 ± 1.2%, with a pEC50 of 7.4 ± 0.1 (Fig. 2, P = 0.85). Brodifacoum failed to affect the hyperpolarization produced by THC in AtT20-CB1 cells (control, pEC50 6.4 ± 0.6, maximum change in fluorescence 18 ± 5%; in brodifacoum, pEC50 6.5 ± 0.5, max 18 ± 5%, P = 0.95). In AtT20-CB2 cells THC only produced a small hyperpolarization, the response to 10 µM THC was unchanged in the presence of brodifacoum (6.4 ± 1.2% in control, 7.4 ± 1.8% in brodifacoum, P = 0.65) (Fig. 2). Application of brodifacoum (10 µM) or CP55940 (1 µM) for 5 min produced very small changes in the fluorescence of wild type AtT20 cells, and neither drug affected the response to subsequently applied somatostatin (100 nM), which activates native SST receptors in AtT20 cells (Günther, Culler & Schulz, 2016) (Fig. S1).
Figure 2: Brodifacoum (BFC) effect on CP55940 and Δ9-THC induced hyperpolarization of AtT20 cell expressing CB1 or CB2.
Representative traces showing the change in fluorescence for CP55940 on (A) AtT20-CB1, and (B) AtT20-CB2 in the presence of BFC 1 µM or vehicle. Values are expressed as a percentage of predrug baseline. A reduction in fluorescence indicates a hyperpolarization. Drugs were added for the duration of the bar; the traces are representative of at least five independent experiments. Concentration response curve of hyperpolarization of AtT20-CB1 or AtT20-CB2 cells stimulated with (C), (D) CP55940 or (E), (F) Δ9-THC in the continued presence of either HBSS or BFC. Data represents the mean ± SEM of five independent experiments performed in duplicate. There was no difference in the potency or maximal effect of CP55940 and Δ9-THC between HBSS or in presence of BFC.Inhibition of adenylyl cyclase activity is another significant biological effect of cannabinoid receptor activation. Brodifacoum (300 nM–30 µM) co-applied with forskolin (3 µM) for 10 min did not affect increases in cAMP levels in HEK 293 cells expressing CB1 or CB2 (Fig. 3). Brodifacoum (1 µM) incubation for 5 min also failed to affect the CP55940 inhibition of forskolin-stimulated cAMP elevation. In cells expressing CB1, CP55940 inhibited cAMP with a pEC50 of 7.5 ± 0.3, to a minimum of 52 ± 12% of forskolin alone; in the presence of brodifacoum these were pEC50 7.4 ± 0.2 and minimum of 52 ± 7% of the forskolin response. Brodifacoum also did not affect forskolin-stimulated cAMP levels in HEK293 cells expressing CB2 (Fig. 3), or CP55940 inhibition of cAMP levels (pEC50 in control cells expressing CB2 7.4 ± 0.2, to a minimum of 39 ± 7%; in brodifacoum pEC50 of 7.5 ± 0.1; to a minimum of 45 ± 4%).
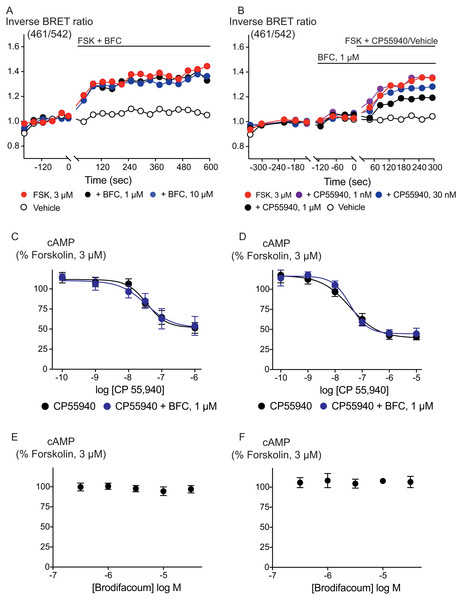
Figure 3: Brodifacoum (BFC) does not modulate cAMP accumulation via CB1 or CB2 receptors expressed in HEK 293 cells.
Representative data from the CAMYEL assay for HEK 293 cells expressing CB1 receptors, an increase in inverse BRET ratio (emission at 461/542 nm) corresponds to an increase in cAMP. (A) BFC does not affect the rapid increase in cAMP production produced by forskolin (3 µM); (B) BFC (1 µM) does not affect responses to forskolin (3 µM) applied in the presence of CP55940. Data are representative of at least five independent experiments. Concentration response curve showing CP55940 induced inhibition of forskolin-stimulated cAMP elevation in presence and absence of BFC 1 µM on HEK 293 cells expressing (C) CB1 or (D) CB2. Data are expressed as a percentage of response produced by forskolin (3 µM), and plotted as mean ± SEM of five independent determinants performed in duplicate. Concentration response curve showing the effect of BFC on forskolin (3 µM)-stimulated cAMP elevations in HEK 293 cells expressing (E) CB1 or (F) CB2. Data are expressed as a percentage of response produced by forskolin (3 µM), and plotted as mean ± SEM of five independent experiments performed in duplicate.We also examined the possibility that brodifacoum could affect the sustained responses to CP55940 or THC. As previously described (Cawston et al., 2013), prolonged application of cannabinoids in AtT20-CB1 cells produces a response that wanes over time, reflecting desensitization of receptor signaling. The degree to which this desensitization reflects changes in signaling specific to cannabinoid receptors is tested by application of somatostatin, which activates receptors native to AtT20 cells (Günther, Culler & Schulz, 2016; Heblinski, Bladen & Connor, 2019). In these experiments, CP55940 (100 nM) or THC (10 µM) were applied 2 min after addition of brodifacoum (1 µM), and the fluorescence monitored for 30 min before the addition of somatostatin (100 nM) (Fig. 4). Desensitization was quantified after 30 min of agonist application, and was expressed as the % decline from the peak response. We did not observe any significant difference in the desensitization of CB1 signaling mediated by CP55940 (100 nM) when co-applied with brodifacoum (Control, 71 ± 4%; brodifacoum treated, 66 ± 7%, P = 0.55). The presence of brodifacoum had no effect on the somatostatin (100 nM) induced hyperpolarization alone, or after 30 mins of CP55940 treatment (P = 0.75) (Fig. S2). The desensitization produced by THC (10 µM, 30 mins) in AtT20-CB1 cells was not different when co-applied with brodifacoum, (Control, 65 ± 6%; brodifacoum treated, 53 ± 8%, P = 0.3) (Fig. 4). A similar reversal of the hyperpolarization produced by CP55940 (100 nM) in AtT20-CB2 cells was also observed. Treatment with brodifacoum did not significantly affect the desensitization produced by CP55940 compared to control cells (Control, 77 ± 6%; brodifacoum treated, 63 ± 8%, P = 0.2). THC (10 µM, 30 mins) signaling at CB2, although modest, also declined during continuous drug exposure, and this was also not affected by co-application of brodifacoum (37 ± 14% in control, 20 ± 7% in brodifacoum treated, P = 0.3) (Fig. 4). The hyperpolarization induced by somatostatin after prolonged application of CP55940 (P = 0.56) or THC (P = 0.87) to AtT20-CB2 cells was also not significantly different in the presence of brodifacoum (Fig. S2).
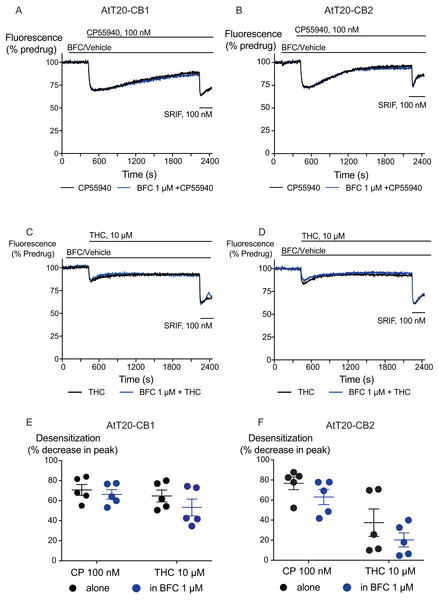
Figure 4: The effect of brodifacoum (BFC) on CP55940 and Δ9-THC mediated desensitization of signalling in AtT20-CB1 and -CB2.
Representative traces showing desensitization of signalling in AtT20-CB1 and AtT20-CB2 on prolonged stimulation with (A), (C) CP55940 (100 nM) or (B), (D) Δ9-THC (10 µM) in the presence of BFC 1 µM or vehicle. Cells were challenged with somatostatin (100 nM) after 30 minutes of CP55940 or Δ9-THC. Drugs were added for the duration of the bar; the traces are representative of at least five independent experiments. Scatter dot plot representing desensitization of (E) CB1 and (F) CB2 on exposure to CP55940 or Δ9-THC for 30 mins in the presence of BFC 1 µM or vehicle. This plot shows percentage desensitization comparing peak fluorescence after the addition of drugs and 30 mins post addition. Data represents the mean ± SEM of five independent experiments performed in duplicate.Discussion
The principal finding of this study is that brodifacoum does not affect CB1 or CB2 signaling, either to K channels in AtT20 cells or adenylyl cyclase in HEK 293 cells. In the assay of K channel activation, there was no effect on the concentration response relationship for CP55940 or THC, and brodifacoum did not affect the desensitization of signaling produced by prolonged application of either drug. Brodifacoum had no effect on the potency, maximum effect or time-dependence of the actions of the high efficacy synthetic cannabinoid CP55940 or the lower efficacy phytocannabinoid THC, indicating that it is unlikely to act as modulator of the pharmacodynamic effects of cannabinoids.
Activation of GIRK is mediated by the Gβγ subunits of G protein heterotrimers, and many Gi/Go coupled receptors effectively signal through this pathway in AtT20 cells (e.g., Mackie et al., 1995; Günther, Culler & Schulz, 2016; Knapman et al., 2013; Heblinski, Bladen & Connor, 2019). We have previously used the fluorescent measurement of membrane potential to study CB1 and CB2 agonists, antagonists, and allosteric modulators of CB1 (Cawston et al., 2013). Inhibition of adenylyl cyclase activity by CB receptors is mediated via the Gα subunits of G protein heterotrimers, and brodifacoum also failed to affect this signal transduction pathway. The precise cellular signaling mechanisms responsible for the subjective effects of Cannabis and synthetic cannabinoid agonists are not established, although the signal transduction of cannabinoid receptors has been extensively studied (Howlett & Abood, 2017; Ibsen, Connor & Glass, 2017) and it is unlikely that any one pathway is responsible. It remains formally possible that brodifacoum could selectively modulate pathways other than Gβγ-mediated activation of GIRK or Gα-mediated inhibition of cAMP accumulation, but the lack of any effect whatsoever on the effects of CP55940 or THC suggests that ligand interactions with cannabinoid receptors are unaffected by brodifacoum.
The concentration of brodifacoum in blood or brain after co-ingestion with synthetic cannabinoids is unknown. However, concentrations of up to 3 µM have been reported in the serum of people who have deliberately ingested large quantities of rat poison (Weitzel et al., 1990; Hollinger & Pastoor, 1993), and inhalation of BFC via smoked synthetic cannabinoids may produce higher serum concentrations of BFC than ingestion. Brodifacoum at 1 µM failed to affect CB1 or CB2 receptor signaling when measured continuously over a period of 30 min, and 10 µM brodifacoum failed to mimic or affect the acute response to a maximally effective concentration of CP 55940, although at this concentration prolonged application of brodifacoum produced a decrease in the fluorescence of wild type AtT20 cells, as well as those expressing CB1 and CB2 receptors. This effect at higher concentrations may reflect direct interactions of brodifacoum with cell membranes (Marangoni et al., 2016). Concentrations of brodifacoum in the upper range of what we tested are achieved only after ingestion of large amounts of rat bait, it is possible that they could be achieved while ingesting contaminated synthetic cannabinoids, but this remains unreported.
Several case reports suggest an interaction between therapeutic warfarin and cannabis or cannabidiol (Grayson et al., 2018; Yamreudeewong et al., 2009; Damkier et al., 2019). It has been suggested that cannabinoid inhibition of enzymes responsible for the metabolism of warfarin can increase blood levels of the drug, and while these studies have focussed on potentially dangerous changes in warfarin concentration, levels of cannabinoids could also be reciprocally elevated. Such interactions may inform a decision to deliberately combine “superwarfarin” with SCRA, as has been previously suggested for cannabis (La Rosa, Clarke & Lefkowitz, 1997; Spahr, Maul & Rodgers, 2007), although whether brodifacoum is metabolized by pathways shared with SCRA in humans is unknown. Information about how or even whether BFC is metabolized in humans is very sparse, although available evidence suggests metabolism is very limited or absent (Hauck, Feinstein & Van Breeman, 2016). Apart from the obvious danger of ingesting brodifacoum, altering the metabolism of SCRA is likely to have unpredictable consequences, as some metabolites of SCRA retain cannabinoid receptor activity (e.g., Brents et al., 2011; Chimalakonda et al., 2012; Longworth et al., 2017; Cannaert et al., 2016), and may contribute to the overall SCRA experience.
Ingestion of brodifacoum is relatively common, while death from exposure is rare, owing to ready treatment with vitamin K (King & Tran, 2015; Gummin et al., 2018). The high number of deaths associated with the combination of SCRA and anticoagulants in 2018 (at least eight; Connors, 2018) may point to an interaction between the drugs. It may also reflect the identity and dose of the synthetic cannabinoid(s) consumed, as well as the general health status of the drug users. Deaths from synthetic cannabinoid exposure are uncommon, but well documented (e.g., Kasper et al., 2015; Trecki, Gerona & Schwartz, 2015). While there is a general acceptance that brodifacoum or a similar agent is responsible for the coagulopathies associated with synthetic cannabinoid ingestion, identification of the synthetic cannabinoid has not been reported in most cases, but a recent report identified a metabolite of AB-FUBINACA in one patient following ingestion of “King Kong”, a brodifacoum laced SCRA (Riley et al., 2019). It seems unlikely, though, that brodifacoum would interact with higher efficacy or potency SCRAs at cannabinoid receptors when it clearly does not interact with CP55940 or THC signaling (Noble et al., 2019; Sachdev et al., in press). Intriguingly, several groups have reported cannabinoid receptor ligands based on a coumarin scaffold (Behrenswerth et al., 2009; Han et al., 2015). While these drugs have been reported to be either antagonists/inverse agonists (Behrenswerth et al., 2009) or CB2-selective agonists (Han et al., 2015), they remain largely uncharacterized. Given the propensity of chemists producing and, in some cases, designing cannabinoids for the recreational market, it cannot be ruled out that coagulopathy may be an unanticipated adverse effect of a synthetic cannabinoid, which may have arisen from a novel, coumarin-based cannabinoid that retains some of the vitamin K epoxide reductase inhibitory of warfarin and brodifacoum.
In conclusion, we report that brodifacoum does not appear to be an agonist or antagonist of human cannabinoid receptors, and it also does not appear to be an allosteric modulator of CB1 or CB2 activation of K channels or inhibition of adenylyl cyclase. Why brodifacoum has been mixed with synthetic cannabinoid receptor agonists remains a matter for speculation, although an intended effect on synthetic cannabinoid drug pharmacokinetics cannot entirely be ruled out.
Supplemental Information
Effects of brodifacoum (BFC) and CP55940 in wild type AtT20 cells
Scatter dot plot representing the percentage change in fluorescence for BFC (30 µM), BFC (10 µM), CP55940 (10 µM), and Vehicle (0.1% DMSO) alone (blue dots), and the response to the subsequent addition of SOMATOSTATIN (100 nM) to AtT20-WT cells (black dots). Data represents the mean ± SEM of five independent experiments performed in duplicate (p ¿0.05).
Effect of brodifacoum (BFC) on somatostatin (SRIF) challenge after 30 minutes of drugs on AtT20-CB1 and -CB2 cells
Comparison of percentage change in fluorescence after SRIF (100 nM) challenge on AtT20-CB1, and AtT20-CB2 in the continuous presence of (A), (C) CP55940 or (B), (D) Δ9-THC added with either HBS or BFC (1 µM). BFC did not affect the hyperpolarization induced by SRIF after prolonged application of CP55940 or Δ9-THC. Data represents the mean ± SEM of five independent experiments performed in duplicate ( p > 0.05).
Data used to generate CRC and dot plots
Each point represents the mean of duplicate determinations. Each point in a series is from an independent experiment.